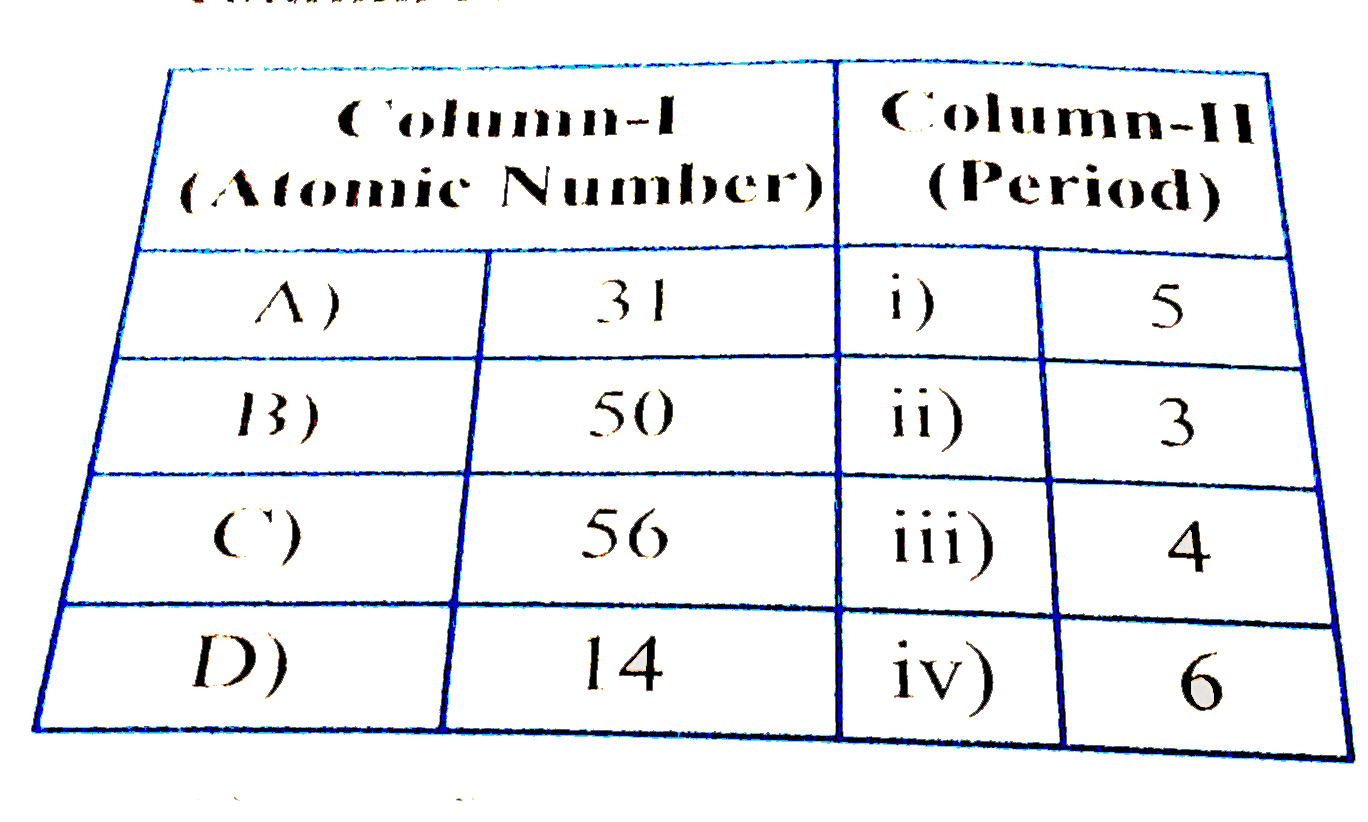
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 63-CHEMISTRY
- Which of the plots is adsorption isobar for chemisorption?
Text Solution
|
- Select the correct option, among Sc(III) , Ti(IV), Pd(II) and Cu(II) i...
Text Solution
|
- Consider the following bromides The correct order of S(N)1 reacti...
Text Solution
|
- Dehydration of the following in increasing order is
Text Solution
|
- The half-life for radioactive decay of .^(14)C is 5730 years . An arch...
Text Solution
|
- Complete the missing links CH(3)CHBrCH(3) overset("alc. KOH")rarr Xo...
Text Solution
|
- The freezing point of 1 molal NaCl solution assuming NaCl to be 100% d...
Text Solution
|
- {:(COOH),(|),(COOH):}overset(NaOH)rarr X, The product (X) will be
Text Solution
|
- NaBH(4)+I(2)rarr X+Y+Z BF(3)+LiAlH(4) overset("450 K")rarr X+P BF(...
Text Solution
|
- In the following reaction, The organic product X has the structur...
Text Solution
|
- Which of the following are arranged in the decreasing order of dipole ...
Text Solution
|
- Study the structure of maltose and mark the incorrect statement.
Text Solution
|
- Work done on a ideal gas in a cylinder when it is compressed by an ext...
Text Solution
|
- Sometimes it is possible to separate two sulphide ores by adjusting th...
Text Solution
|
- Match the atomic numbers of the elements given in column I with the pe...
Text Solution
|
- The drain cleaner Drainex contains small bits of aluminium which react...
Text Solution
|
- In the complex K(4)[Th(C(2)O(4))(2)(H(2)O)(2)]. If coordination number...
Text Solution
|
- How many these compounds/Ions are aromatic here?
Text Solution
|
- How many of these metals can displace H(2) easily from acids. Fe, Mg...
Text Solution
|
- How many of these carbocations are more stable than (CH(3))(3)C^(+) ...
Text Solution
|