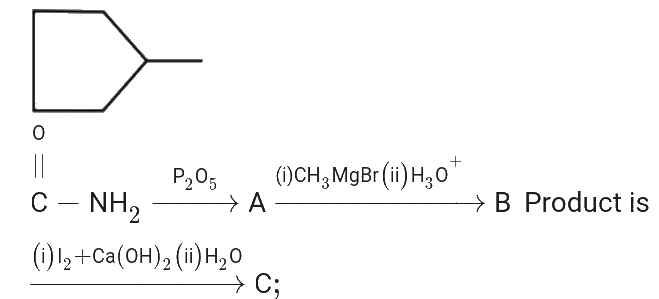
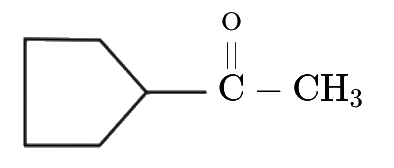
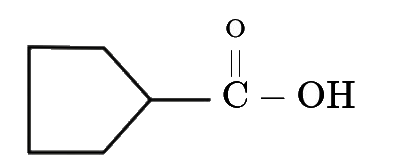
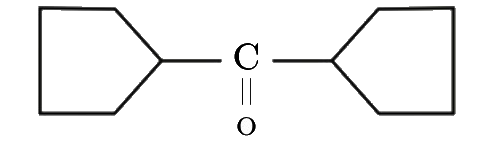
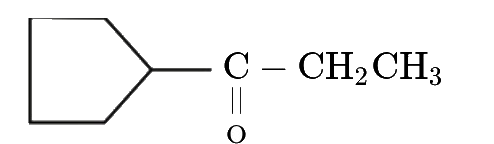
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
NTA MOCK TESTS-NTA JEE MOCK TEST 69-CHEMISTRY
- Consider the following compound (A). Select the correct statement.
Text Solution
|
- Find the final product of the reaction
Text Solution
|
- [XeO(6)]^(4-) is octahedral whereas XeF(6) is a disordered one, becaus...
Text Solution
|
- Which of the following is least basic?
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- A+H(2)O to B+HCl B+H(2)O to C+HCl Compound (A), (B) and (C) will b...
Text Solution
|
- In assigning R-S configuration which among the following groups has hi...
Text Solution
|
- The pH of blood stream is maintained by a proper balance of H(2)CO(3) ...
Text Solution
|
- A metal on combustiion in excess air forms X.X upon hydrolysis with wa...
Text Solution
|
- An electron practically at rest, is initially accelerated through a po...
Text Solution
|
- In which of the following reaction CO(2) (carbon dioxide) is not relea...
Text Solution
|
- The helical structure of protein is stabilized by
Text Solution
|
Text Solution
|
- The reaction that is NOT involved in the ozone layer depletion mechani...
Text Solution
|
- The major product (X) of the monobromination reaction is
Text Solution
|
- For the reaction A hArr B+C at equilibrium, the concentration of A is ...
Text Solution
|
- How many of these acids have S-S bonds? H(2)S(2)O(3), H(2)S(2)O(6), ...
Text Solution
|
- The number of geometrical isomers of the compound is C(6)H(5)-CH=CH-...
Text Solution
|
- Perovskite is a mineral composed of Ca, Ti and oxygen, cations of tita...
Text Solution
|
- How many of these carbocations are more stable than (CH(3))(3)C^(+) ...
Text Solution
|