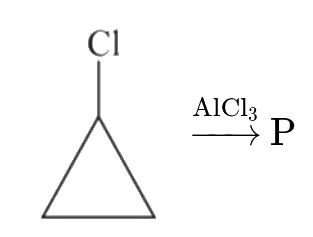
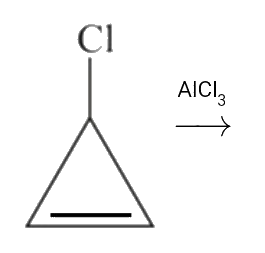
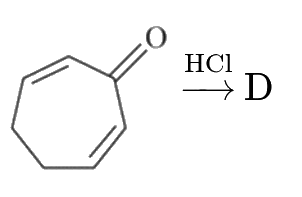
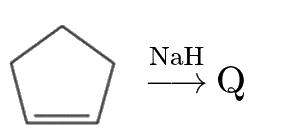A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 71-CHEMISTRY
- Based on the first law of thermodynamics which one of the following is...
Text Solution
|
- e.m.f. diagram for some ions is given as : FeO(4)^(2-)overset(E^(@)=...
Text Solution
|
- Among P, Q, R, S the aromatic compound is
Text Solution
|
- Consider two reactions having same Arrhenius factor A, but different e...
Text Solution
|
- The correct name of the structure
Text Solution
|
- Which of the following cannot give Cannizzaro reaction?
Text Solution
|
- In a solid, oxide ions are arranged in ccp. Cations 'A' occupy one - s...
Text Solution
|
- Aspirin is an acetylation product of :
Text Solution
|
- Phenylacetylene on treatment with HgSO(4)//H(2)SO(4), H(2)O produces
Text Solution
|
- The ions present in Al(4)C(3), CaC(2) and Mg(2)C(3) are respectively
Text Solution
|
- If 200 ml of 0.031 M solution of H (2) SO(4) is added to 84 ml of a 0....
Text Solution
|
- The correct statement for the following addition reaction is
Text Solution
|
- Consider the following reactions, overset(Br(2)//FeBr^(3))toPoverse...
Text Solution
|
- What will be product of the following given reaction ?
Text Solution
|
- The elements X and Y form compound having molecular formula XY(2) and ...
Text Solution
|
- The wave function orbital of H-like atoms is given as onder psi(2s)...
Text Solution
|
- The number of geometrical isomers possible for the complex [Pt(NH(3))(...
Text Solution
|
- 0.7 g " of " Na(2)CO(3).xH(2)O were dissolved in water and the volume ...
Text Solution
|
- Maximum no. of moles of gridnard reagent that can be used is x. Find x...
Text Solution
|
- underset((A))(C(12)H(16))overset("i) "O(3)"ii) "Zn,H(2)O(2))rarr 2H(3)...
Text Solution
|