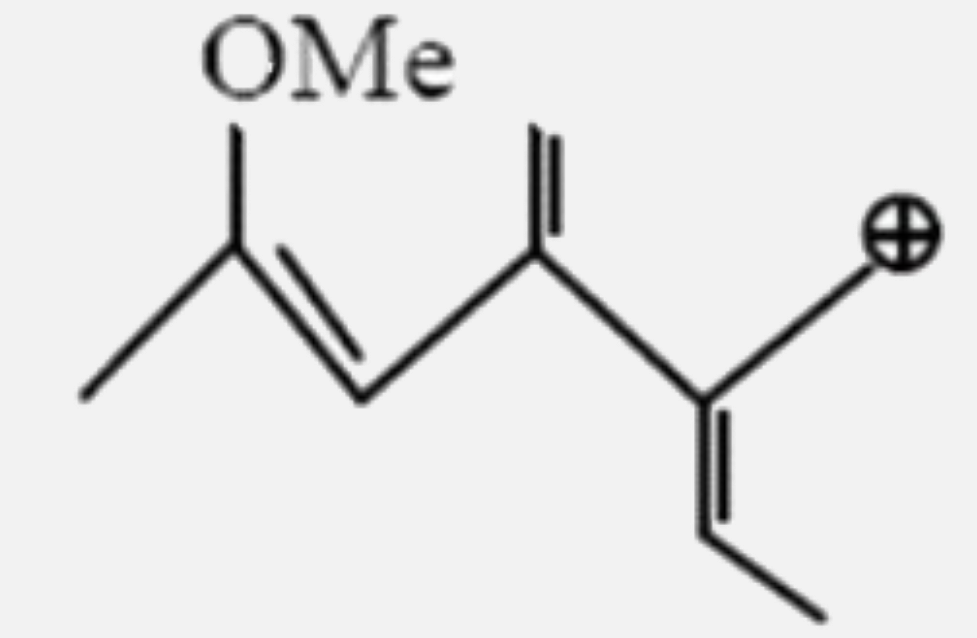
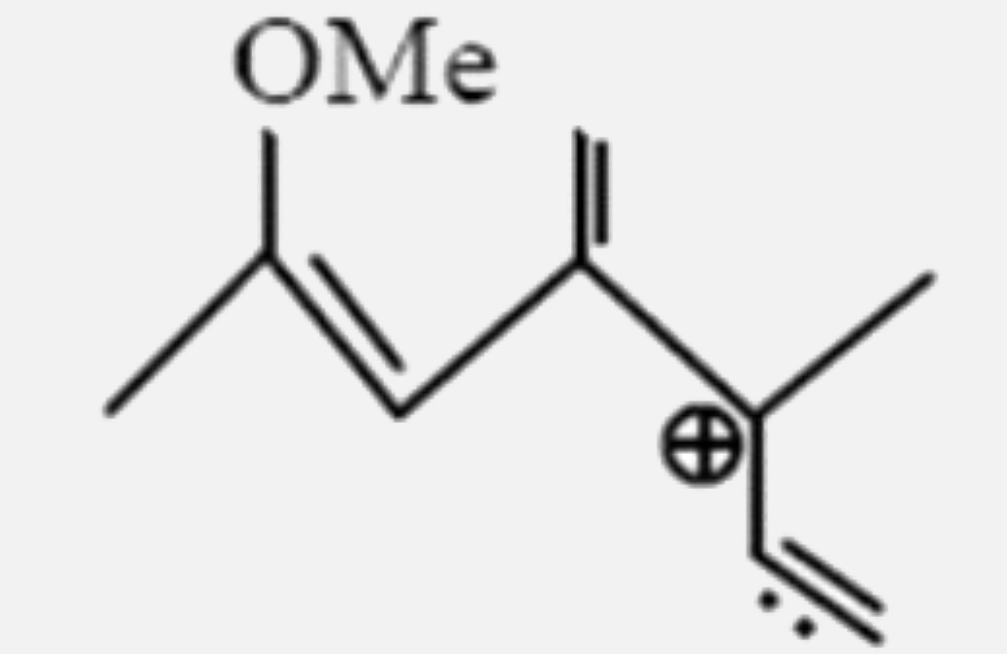
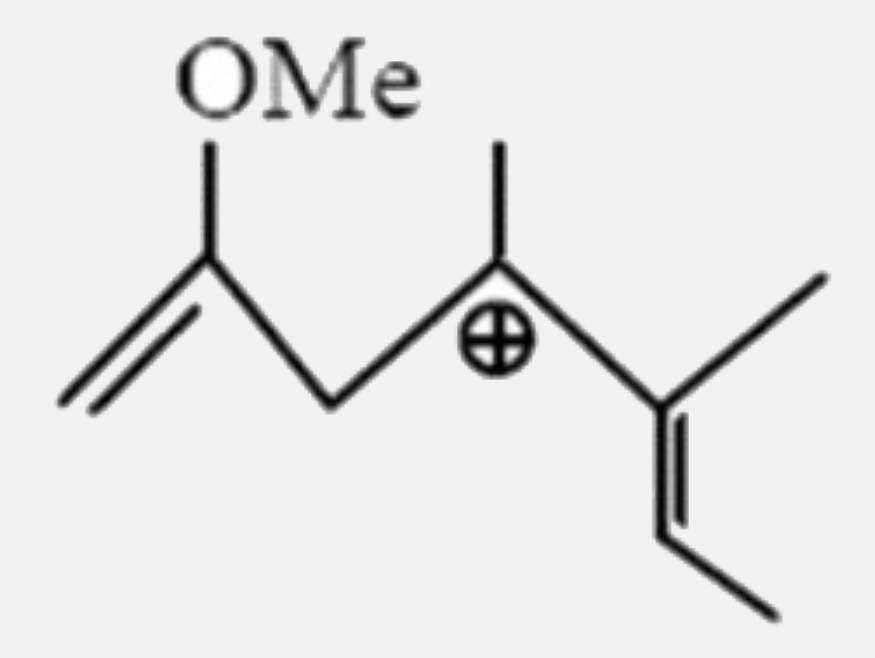
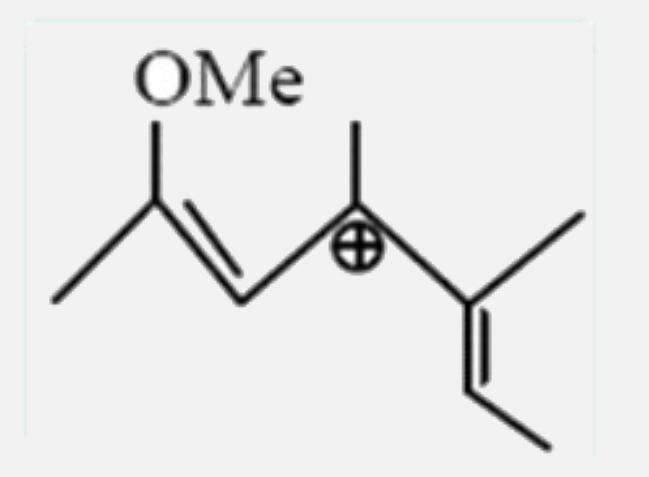
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 19-CHEMISTRY
- In the reaction sequence C6H5CHOoverset(NaCN//HCl)rarr(X)overset(H2"O"...
Text Solution
|
- Prussian blue is -
Text Solution
|
- F-centers are
Text Solution
|
- Amongest H(2)O,H(2)S,H(2)Se and H(2)Te the one with highest boiling p...
Text Solution
|
- Among KO(2), KAlO(2), CaO(2) and NO(2)^(+), unpaired electrons is pres...
Text Solution
|
- An organic molecule necessarily shows optical acitivity if it
Text Solution
|
- Microcosmic salt reacts with coloured ions to form characteristic bead...
Text Solution
|
- CH(3)-overset(CH(3))overset("| ")underset(CH(3))underset("| ")"C "-C...
Text Solution
|
- Which of the following sequence represents the correct increasing orde...
Text Solution
|
- (CH(3))(2)C=CHCOCH(3) can be oxidised to (CH(3))(2) C=CHCOOH by
Text Solution
|
- Select the most stable carbocation:
Text Solution
|
- Zeolites are extensively used in -
Text Solution
|
- Which of following statements is false ?
Text Solution
|
- Consider the following statements and choose the correct option (i) ...
Text Solution
|
- Total vapour pressure of mixture of 1 mol volatile component A(P^(@)A=...
Text Solution
|
- The correct order of bond dissociation energies of various C-H bonds p...
Text Solution
|
- Calculate the number of oxygen atoms required to ccombine with 7 g of ...
Text Solution
|
- What is the major product in the following reaction :
Text Solution
|
- Which of the following is an elastomer?
Text Solution
|
- Select the incorrect statement.
Text Solution
|