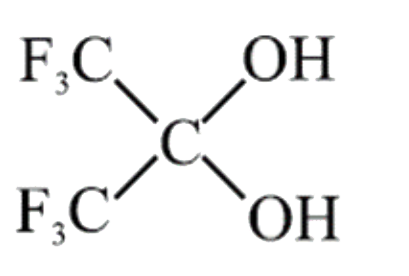
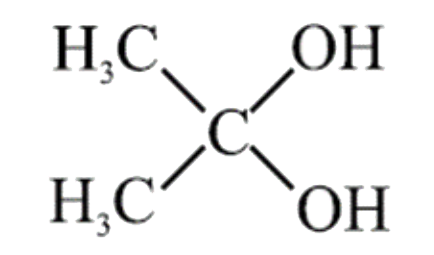
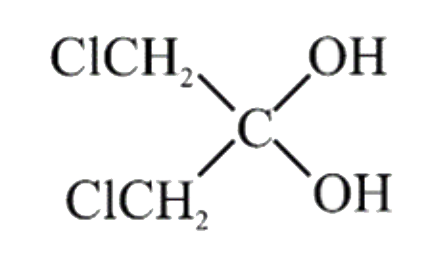
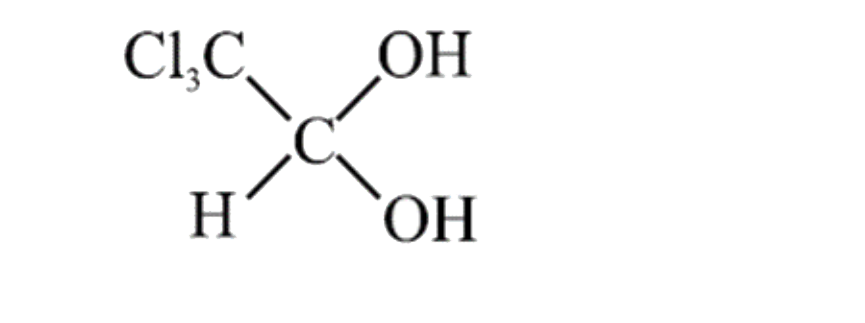
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET TEST 22-CHEMISTRY
- Which of the following is not present in nucleotide?
Text Solution
|
- Molar conductivity of a solution of an electrolyte AB3 is 150Scm^2mol^...
Text Solution
|
- Which of the following geminal diols is most unstable:
Text Solution
|
- The standard enthalpy of neutralization of strong acid and strong base...
Text Solution
|
- In a reversible adiabatic change Delta Q is
Text Solution
|
- Which of the following pair of species have identical shape?
Text Solution
|
- CH3-overset(OH)overset(|)(CH)-overset(O)overset(||)(C )-CH2 - CH(3) wi...
Text Solution
|
- Given that equilibrium constant for the reaction 2SO(2)(g) + O(2)(g)hA...
Text Solution
|
- Which is a pair of geometrical isomers?
Text Solution
|
- The temperature of a sample of a gas is raised from 127^(@)C to 527^(@...
Text Solution
|
- In the radioactive decay of ""(Z)X^(A), which of the following could b...
Text Solution
|
- Give the IUPAC name of m - ClCh2C6H4CH2C(CH3)(3)
Text Solution
|
Text Solution
|
- Benzene and toulene form an ideal solution. 3 mole benzene and 2 mole ...
Text Solution
|
- Which of the following oxides is strongly basic?
Text Solution
|
- Amongst NO(3)^(-), AsO(3)^(3-), CO(3)^(2-),ClO(3)^(-),SO(3)^(2-) and B...
Text Solution
|
- Which of the following is hypnotic drug?
Text Solution
|
- Calculate Q and w for the isothermal reversible expansion of one mole ...
Text Solution
|
- If all the electrolytes removed from the colloid by persistent dialysi...
Text Solution
|
- The correct order of increasing basic nature of the bases NH(3),CH(2)N...
Text Solution
|