A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET TEST 25-CHEMISTRY
- Choose the incorrect match :
Text Solution
|
- Which of the following solutions will have the highest boiling point?
Text Solution
|
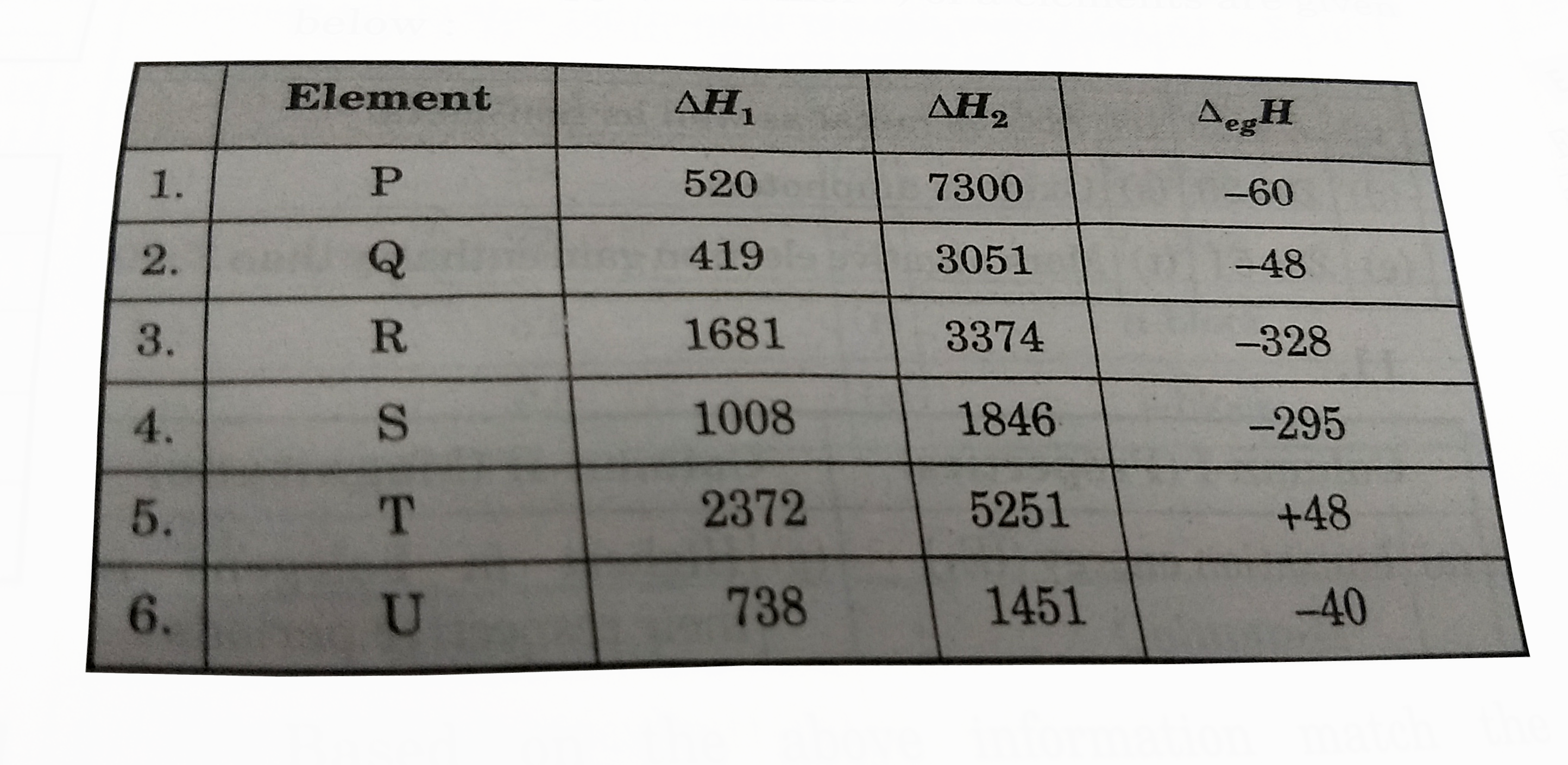
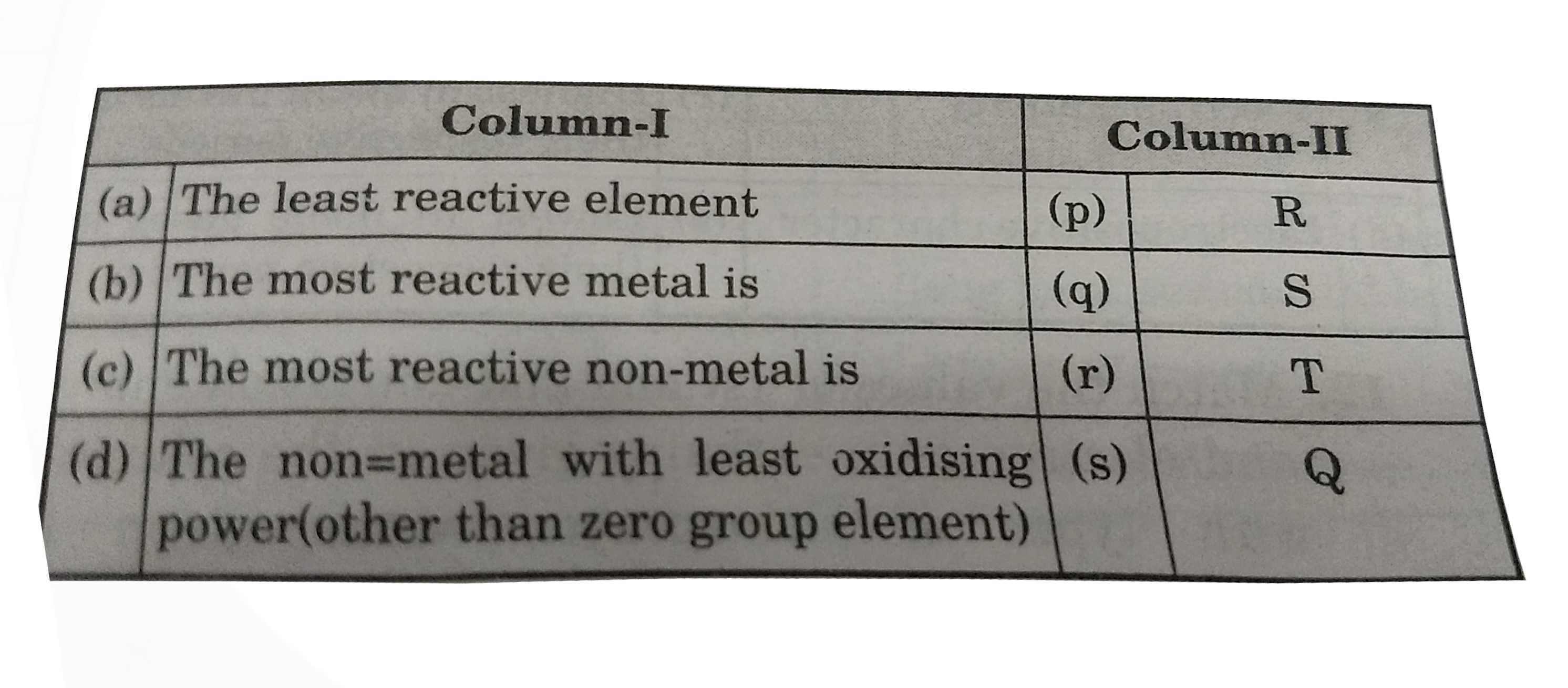
- The first (DeltaH(1)) and second (DeltaH(2)) ionisation enthalpies (in...
Text Solution
|
- Which of the following undergoes reduction with H(2)O(2) in an alkalin...
Text Solution
|
- The metal that produces red violet colour in the non - luminous flame ...
Text Solution
|
- Among the oxyacids of phosphorous the dibasic acid is
Text Solution
|
- Mark the correct statements(s) (1) Manganeses exhibits +7 oxidation ...
Text Solution
|
- Which among the following statement are true for the complex [Co(NH(...
Text Solution
|
- In the balanced chemical reaction IO(3)^(ө)+aI^(ө)+bH^(ө)rarrcH(2)O+...
Text Solution
|
- Halogens exist in -1, +1, +3, +5 and +7 oxidation states. The halogen ...
Text Solution
|
- In petrochemical industry , alcohols are directly converted to gasolin...
Text Solution
|
- In Lassaigne's test for the detection of halogen , the sodium fusion e...
Text Solution
|
- All carbon atoms are sp^(2) hybridised in
Text Solution
|
- Which if the following pairs of compound is a ring chain isomer ?
Text Solution
|
- For two weak acids A and B, the ratio of their percent ionization is 4...
Text Solution
|
- The maximum number of carbon atoms arranged linearly in the molecule, ...
Text Solution
|
- The number of atoms in a cubic based unit cell having one atom on each...
Text Solution
|
- The IUPAC name of the following compound is (CH(3))(2)CH-CH(2)CH=CH-...
Text Solution
|
- Chlorination of benzene in the presence of halogen carrier is an examp...
Text Solution
|
- Aryl halides do not undergo uncleophilic substitution reactions under ...
Text Solution
|