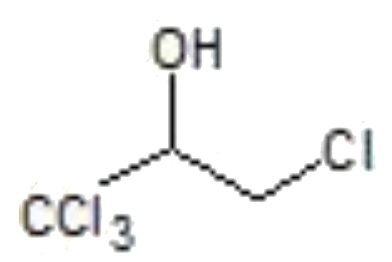
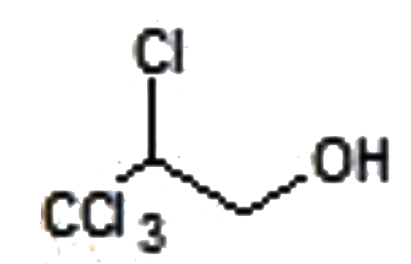
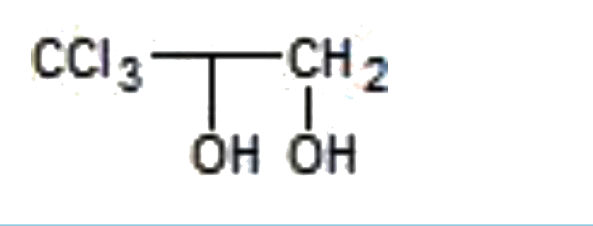
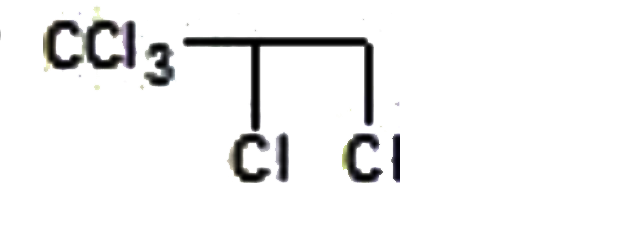A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 29-CHEMISTRY
- CH(3)COOH overset(Br(2)//P)rarr Y underset((i) H(3)O^(+))overset((i)KC...
Text Solution
|
- Ph-CH2-C-=CHunderset(Y)overset(X)hArrPh-C-=C-CH3. The reagents X an...
Text Solution
|
- "CCl"3CH=CH2overset(Cl2+H2O)rarrA, is
Text Solution
|
- In Dumas method 0.5 g of an organic compound containing nitrogen gave ...
Text Solution
|
- 50 mL of 10 N H(2)SO(4), 25 mL of 12 N HCI and 40 mL of 5N HNO(3) are ...
Text Solution
|
- The major product of the reaction is
Text Solution
|
- The van der Waals' constant 'b' of a gas is 4pixx 10^(-4)L//mol. How n...
Text Solution
|
- Metal ions like Ag^(+),Cu^(2+) etc. act as
Text Solution
|
- pH of a saturated solution of Ca(OH)(2) is 9. the solubility product (...
Text Solution
|
- Complete the following reactions, (i) H2(g)+MnO(s)overset(Delta)rar...
Text Solution
|
- The average kinetic energy of one molecule of an ideal gas at 27^@C an...
Text Solution
|
- The colour of CuCr2 O7 solution in water is green because
Text Solution
|
- The number of structural and configurational isomers of a bromo compou...
Text Solution
|
- Determine the oxidation number of the underlined atom is (NH4)(6)ul(M...
Text Solution
|
- Which of the following concentration processes will you use when the g...
Text Solution
|
- 0.15g of a substance dissolved in 15g of solvent boiled at a temperatu...
Text Solution
|
- In a compound AB, electro negativity difference between A and B is 1.9...
Text Solution
|
- In a chemical reaction , a catalyst used for
Text Solution
|
- The least number of oxyacids are formed by:
Text Solution
|
- Ethylidene chloride on treatment with aqueous KOH gives .
Text Solution
|