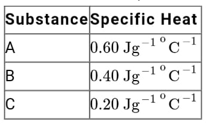
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 31-CHEMISTRY
- Which of the following compounds will not give Lassaigne's test for ni...
Text Solution
|
- How many grams of ice at 0^@C can be melted by the addition of 500 J o...
Text Solution
|
- A 1.0g sample of substance A at 100^(@)C is added to 100 mL of H(2)O ...
Text Solution
|
- By what factor does the average velocity of a gaseous molecule increas...
Text Solution
|
- When solid lead iodide is added to water, the equilibrium concentratio...
Text Solution
|
- The free energy of formation of NO is 78 kJ mol^(-1) at the temperatur...
Text Solution
|
- The first order reaction 2N(2)O(g)rarr2N(2)(g)+O(2)(g) has a rate cons...
Text Solution
|
- What will happen to volume of a bubble of air found under water in a l...
Text Solution
|
- What is the [H^(+)] in 0.40 M solution of , HOCl , Ka = 3.5xx10^(-8) ?
Text Solution
|
- Sodium chloride , NaCl , usually crystallizes in a face - centered cub...
Text Solution
|
- What is the osmotic pressure of a 0.0020 mol dm^(-3) sucrose (C(12)H(2...
Text Solution
|
- Calculate the wavelength of light required to break the bond between t...
Text Solution
|
- As O2 (I) is cooled at 1 atm pressure , it freezes to form solid I at ...
Text Solution
|
- Which of the following configuration of ions has zero CFSE in both str...
Text Solution
|
- Pi (pi) bond is formed by the overlap of
Text Solution
|
- Which of the following complex has minimum magnitude of Delta^(0) ?
Text Solution
|
- The polarity of the covalent bond among the following is maximum in
Text Solution
|
- Which of the following ions gives coloured solution?
Text Solution
|
- On adding excess of NH4OH to copper sulphate solution
Text Solution
|
- The bond angle formed by different hybrid orbitals are in the order
Text Solution
|