A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 33-CHEMISTRY
- Substances used in bringing down the body temperature in high fevers a...
Text Solution
|
- Determine the oxidation number of the underlined atom in Rb4Na[HVul(10...
Text Solution
|
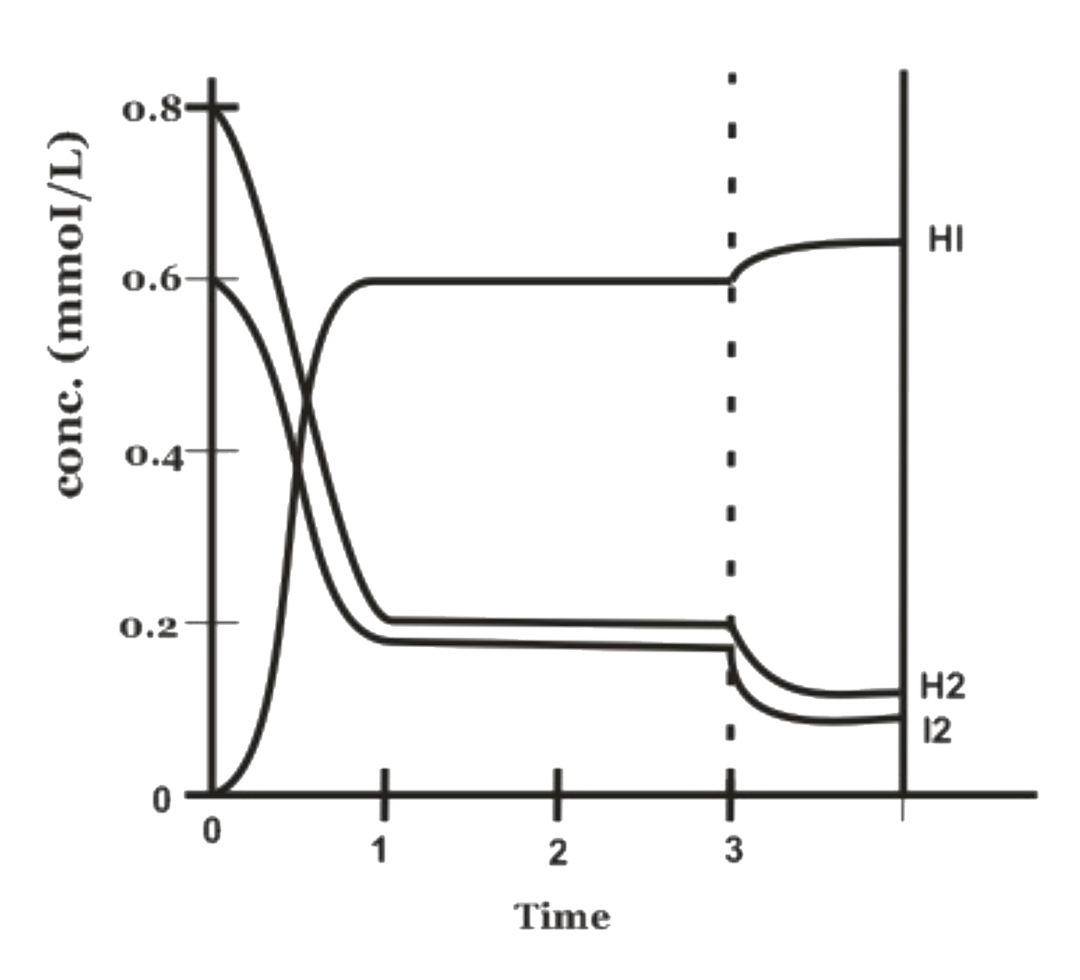
- The equation for the reaction in the figure below is : H2(g)+i2(g)+"...
Text Solution
|
- The elements having the maximum and minimum melting points among the m...
Text Solution
|
- A solution which is 10^(-3) M each in Mn^(2+),Fe^(2+),Zn^(2+) and Hg^(...
Text Solution
|
- For the given reaction , A+Brarr Products Following data are given...
Text Solution
|
- The reagent needed for converting is :
Text Solution
|
- One mole of an ideal gas (C(V) = 20 JK^(-1) mol^(-1)) initially at STP...
Text Solution
|
- Total number of geometrical isomers for the complex [Rh Cl (CO) (PPh(3...
Text Solution
|
- The correct sequence of decreasing number of pi - bonds in the structu...
Text Solution
|
- Which property of colloids is not dependent on the change on colloidal...
Text Solution
|
- In a closed vessel of 5 litres capacity, 1 g of O(2) is heated from 3...
Text Solution
|
- The IUPAC name of
Text Solution
|
- What product (s) is (are) obtained when 2 - bromobutane undergoes an e...
Text Solution
|
- The radius of La^(3+)(Z=57) is 106 pm. Which one of the following give...
Text Solution
|
- Alcohols react with Grignard reagent to form
Text Solution
|
- The aqueous solution of D - glucose contains two forms of D - glucopyr...
Text Solution
|
- What volume of water is required to make 0.20N solution from 1600 mL o...
Text Solution
|
- Adsorbed hydrogen by Palladium is known as
Text Solution
|
- Chromatography was discovered by
Text Solution
|