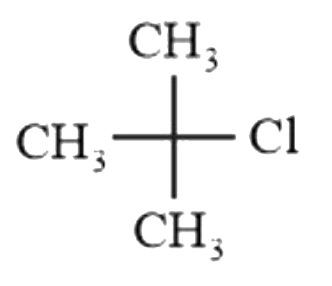
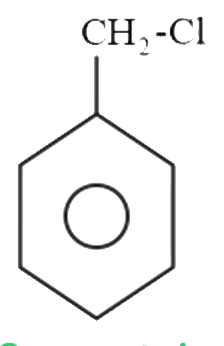
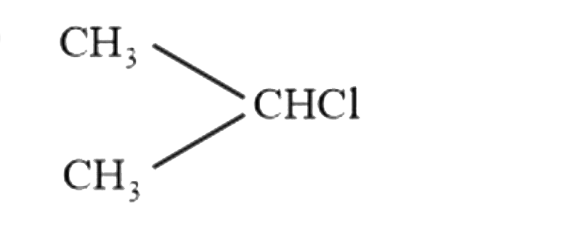
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 40-CHEMISTRY
- Which of these statements about [Co(CN)6]^(3-) is true?
Text Solution
|
- The reaction of H(3)N(3)B(3)Cl(3)(A) with LiBH(4) in tetrahydrofuran g...
Text Solution
|
- In which of the following compounds , the C - Cl bond ioniosation shal...
Text Solution
|
- In the following reaction A is
Text Solution
|
- Consider the following compounds Hyper conjugation occurs in
Text Solution
|
- The reaction CH3-underset(CH3)underset(|)overset(CH3)overset(|)C-ONa+C...
Text Solution
|
- The anodic half-cell of lead-acid battery is recharged using electrici...
Text Solution
|
- The tests performed on compound X and their inferences are : {:(" ...
Text Solution
|
- When propyne is treated with aqueous H2SO4 in the presence of HgSO4, t...
Text Solution
|
- A compound 'X' on treatment with Br(2)//NaOH, provided C(3)H(9)N, whi...
Text Solution
|
- Among the colloids cheese (C), milk (M) and smoke (S), the correct com...
Text Solution
|
- An organic compound contains C = 40%, H = 13.33%, and N = 46.67%. Its ...
Text Solution
|
- The major product of the following reactions
Text Solution
|
- For the reaction : H(2)+I(2)to 2HI, the differential rate law is
Text Solution
|
- Given The enthalpy of the hydrogenation of these compounds will ...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Dissolving 120g of urea (mol wt = 60) in 1000g of water gave a solutio...
Text Solution
|
- Which polymer has a 'chiral' monomer (s) ?
Text Solution
|
- Bithional is generally added to the soaps as an additive to function a...
Text Solution
|
- Consider the following sequence of reaction The final product (Q) i...
Text Solution
|