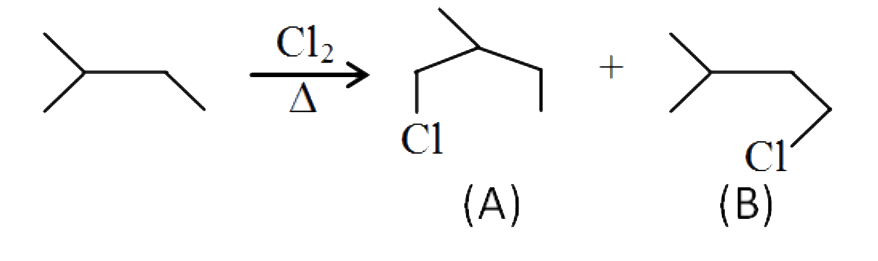
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 42-CHEMISTRY
- The equilibrium that exists in aqueous solution ,CH3COOHhArrCH3COO^(-)...
Text Solution
|
- For the reaction of one mole of zinc dust with one mole of sulphuric a...
Text Solution
|
- The rate of a non - geseous reaction does not dependent on
Text Solution
|
- In the given cell representation Zn|Zn^(2+)|| Cu^(2+)|Cu , which is th...
Text Solution
|
- In the following reaction correct change in phosphorus is explained b...
Text Solution
|
- What is the oxidising agent in chlorine water ?
Text Solution
|
- Activated charcoal is used to remove colouring matter from pure substa...
Text Solution
|
- P(2)O(5) is heated with water to give
Text Solution
|
- One electron species having ionization enegry of 54.4 eV is
Text Solution
|
- The structure of compound, which is formed by sp^3d hybridization will...
Text Solution
|
- Arrange following carbocation in the decreasing order of stability (...
Text Solution
|
- Which is not an example of an ideal solution ?
Text Solution
|
- Calculate the heat of formation of CO using given equations C+O2rarr...
Text Solution
|
- Calculate the standard potential of the cell ,If the standard electrod...
Text Solution
|
- The total number of structural ethers possible with the molecular form...
Text Solution
|
- What is vinegar ?
Text Solution
|
- Which of the following polymer is an example of fibre ?
Text Solution
|
- The gas A is bubbled through lime water , a while precipitate is forme...
Text Solution
|
- If the reactivity factor for chlorine substitution through free radica...
Text Solution
|
- The missing structures A and B in the recation sequence: R -CH(2)-...
Text Solution
|