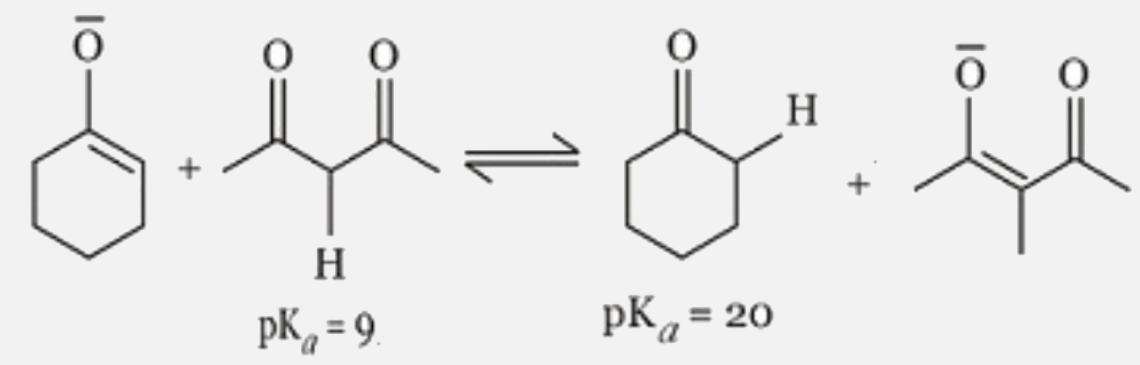
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 46-CHEMISTRY
- Equivalent weight of crystalline oxalic acid is
Text Solution
|
- The reaction , M^(2+) (aq) +M(s) hArr 2M^+(aq) is an example of :
Text Solution
|
- The equilibrium constant for the following reaction is
Text Solution
|
- Which of the following is paramagnetic
Text Solution
|
- Which of the following is not an organometallic compound ?
Text Solution
|
- Choose the correct answer. A thermodynamic state function is a quantit...
Text Solution
|
- An open flask containing air is heated from 300K to 500K. What percent...
Text Solution
|
- The IUPAC name of the compound having the formula is H3C-underset(C...
Text Solution
|
- A solution of metal hydroxide (MOH) with copper sulphate and mixed tar...
Text Solution
|
- A quantity of PCI 5 was heated in a 2 litre vessel at 525 K . It disso...
Text Solution
|
- When aqueous NaOH is added to an aqueous solution of chromium (III )...
Text Solution
|
- Which product is formed when the following compound is treated with B....
Text Solution
|
- The interatomic distance in H(2) and CI(2) molecules are 74 an d198 pm...
Text Solution
|
- Which of the following is not a broad spectrum antibiotic?
Text Solution
|
- Van Arkel method of purification of metals involves converting the met...
Text Solution
|
- The complex showing a spin - only magnetic moment
Text Solution
|
- The chemical formula of feldspar is
Text Solution
|
- Determine the relationship between the two compounds :
Text Solution
|
- 1.00 g of a non-electrolyte solute dissolved in 50g of benzene lowered...
Text Solution
|
- Molish's test is answered by
Text Solution
|