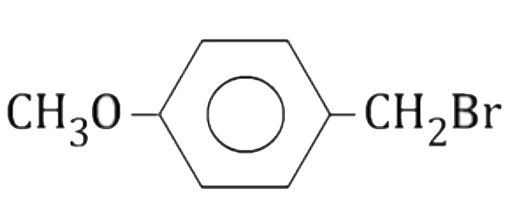
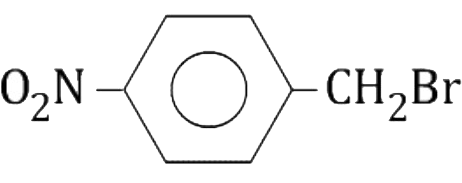
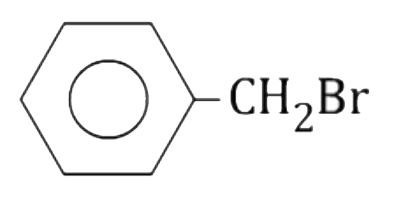
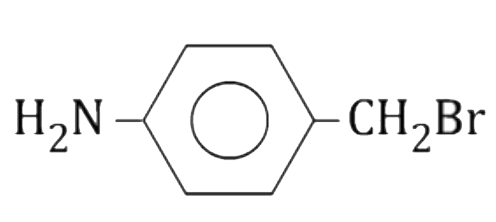
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 50-CHEMISTRY
- The cyanide ion CN and N(2) are isoelectronic, but in contrast to CN^(...
Text Solution
|
- Which of the following types of forces bind together the carbon atoms ...
Text Solution
|
- Which one of the following compounds undergoes predominantly SN^2 reac...
Text Solution
|
- Total charge required for the oxidation of two moles Mn3O4 into MnO4^...
Text Solution
|
- When heated , ammonium carbamate decomposes as follows NH4 COONH2 (s) ...
Text Solution
|
- Which one of the following ionic species will impart colour to an aque...
Text Solution
|
- The reaction of (S) - 2 - bromobutane with OH^- to produce (R) - butan...
Text Solution
|
- Calculate elevation in boiling point for 2 molal aqueous solution of g...
Text Solution
|
- CH3-CH2-underset((X))(CH2)-=N,CH3-underset((Y))underset(CN)underset(|)...
Text Solution
|
- Which of the compounds HCHO(I),CH3CH2CHO(II),CH3COCH3(III) and HCOOC2H...
Text Solution
|
- Solubility of calcium phosphate (molecular mass, M) in water is W g pe...
Text Solution
|
- Select the correct statement
Text Solution
|
- In the following reactions, the major product W is
Text Solution
|
- Galvanization is applying a coating of :
Text Solution
|
- When dihydroxy acetone reacts with HIO(4), the product is /are
Text Solution
|
- The hottest region of Bunsen flame shown in the figure below is :
Text Solution
|
- Select incorrect order
Text Solution
|
- The IUPAC name of
Text Solution
|
- Which one of these is not an acid salt ?
Text Solution
|
- The compressibility factor of gases is less than unity at STP. Therefo...
Text Solution
|