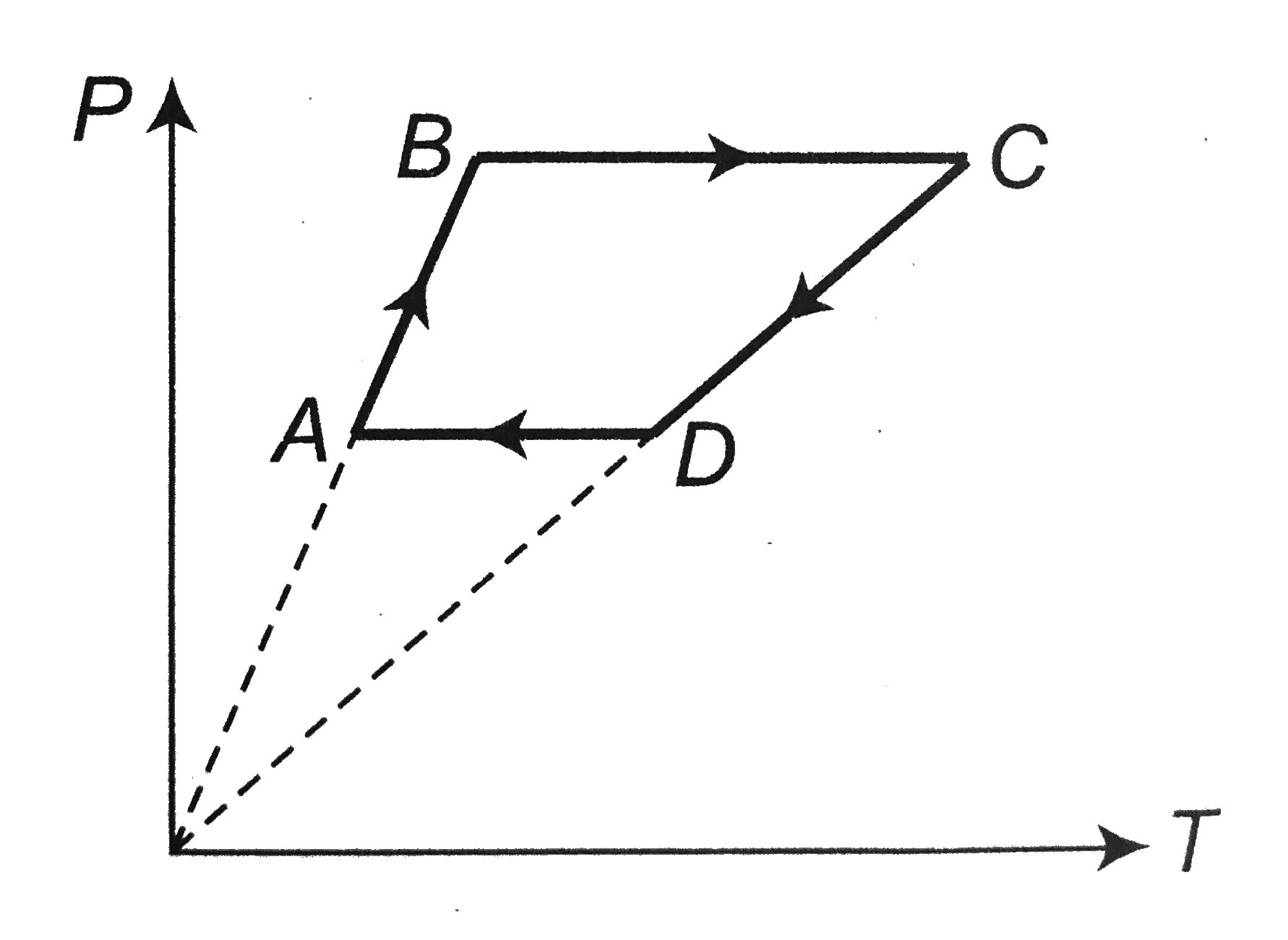
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 54-PHYSICS
- A diatomic ideal gas undergoes a thermodynamic change according to the...
Text Solution
|
- A cyclic process ABCA is shown in the V - T diagram . Process on the P...
Text Solution
|
- Six moles of an ideal gas performs a cycle shown in figure. If the tem...
Text Solution
|
- A metal ball immersed in water weighs w(1) at 0^(@)C and w(2) at 50^(@...
Text Solution
|
- If there is no heat loss, the heat released by the condensation of x g...
Text Solution
|
- Both strings shown in figure, are made of same material and have same ...
Text Solution
|
- The motion of a Particle moving along then y- axis is represented as y...
Text Solution
|
- A particle is projected with speed 20m s^(-1) at an angle 30^@ With h...
Text Solution
|
- A particle is projected along the line of greatest slope up a rough pl...
Text Solution
|
- A 1.0 kg ball drops vertically into a floor from a height of 25 cm . I...
Text Solution
|
- An object comprises of a uniform ring of radius R and its uniform chor...
Text Solution
|
- A man of mass 80 kg is riding on a small cart of mass 40 kg which is r...
Text Solution
|
- A ball moving on a horizontal frictionless plane hits an identical bal...
Text Solution
|
- Keeping the banking angle same , to increase the maximum speed with wh...
Text Solution
|
- If the kinetic energy of a satellite orbiting around the earth is doub...
Text Solution
|
- Two satellite A and B , ratio of masses 3 : 1 are in circular orbits o...
Text Solution
|
- To study the dissipations of the energy of a simple pendulum, student ...
Text Solution
|
- If the same weight is suspended from three springs having length in th...
Text Solution
|
- A U-tube of base length l filled with the same volume of two liquids o...
Text Solution
|
- The work done to get n smaller equal size spherical drops from a bigge...
Text Solution
|