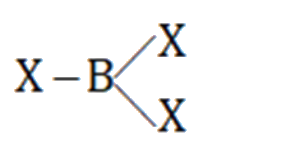A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 61-CHEMISTRY
- The temperature coefficient of a reaction is 2. When the temperature i...
Text Solution
|
- 100 mL of a solution with pH = 6 is diluted to 1000 mL by adding water...
Text Solution
|
- In BX3,B-X distance is shortes than what is expected theoretically bec...
Text Solution
|
- A gaseous hydrocarbon gives upon combustion 0.72g of water and 3.08g o...
Text Solution
|
- Which of the following represents the correct order of increasing firs...
Text Solution
|
- What type of forces bind the substrate to the active site of enzyme ? ...
Text Solution
|
- Which of the following has the highest nucleophilicity ?
Text Solution
|
- The molarity of a solution obtained by mixing 750 mL of 0.5 (M) HCl wi...
Text Solution
|
- An explosion takes place when conc. H2SO4 is added to KMnO4. Which of ...
Text Solution
|
- During the fusion of an organic compound with sodium metal, nitrogen o...
Text Solution
|
- In a buffer solution consisting of a mixture of weak base and its salt...
Text Solution
|
- At 37^@C , the osmotic pressure of blood is 8.21 atm . The amount of g...
Text Solution
|
- The geometries of the ammonia complexes of Ni^(2+),Pt^(2+) and Zn^(2+)...
Text Solution
|
- The oxidation number of sulphur in S(8), S(2)F(2) and H(2)S respective...
Text Solution
|
- In the following reaction, is
Text Solution
|
- When PbO2 reacts with conc HNO3, the gas (es ) evolved is//are .
Text Solution
|
- The number of possible organobromine compounds which can be obtained i...
Text Solution
|
- The correct increasing order of solubility among the compounds follows...
Text Solution
|
- Which of the following statement is false for alkali metals ?
Text Solution
|
- Which will be the correct stability order of the different conformatio...
Text Solution
|