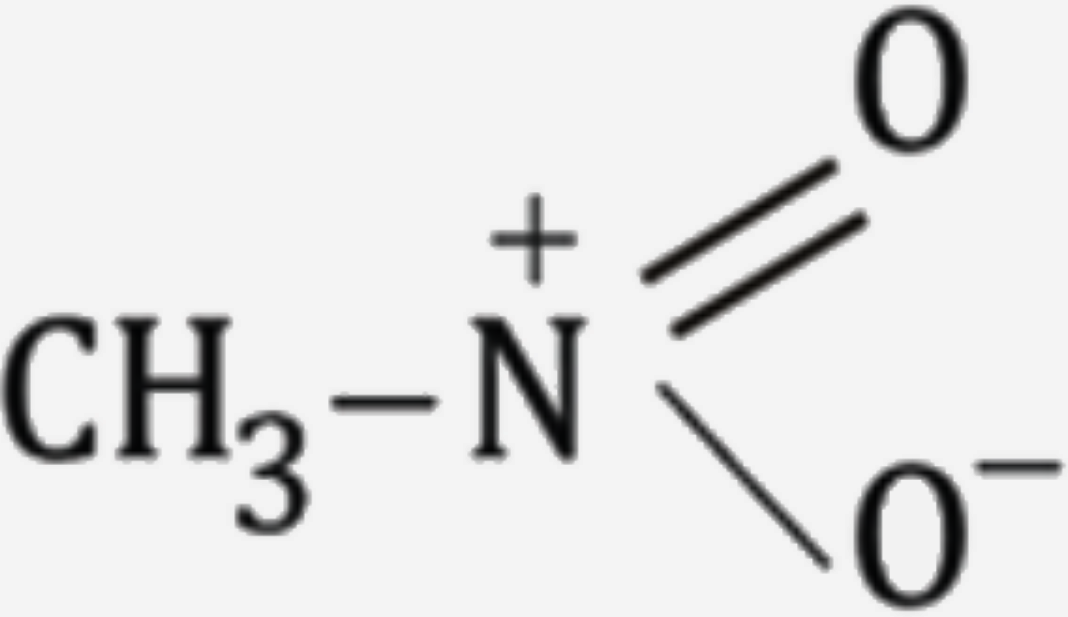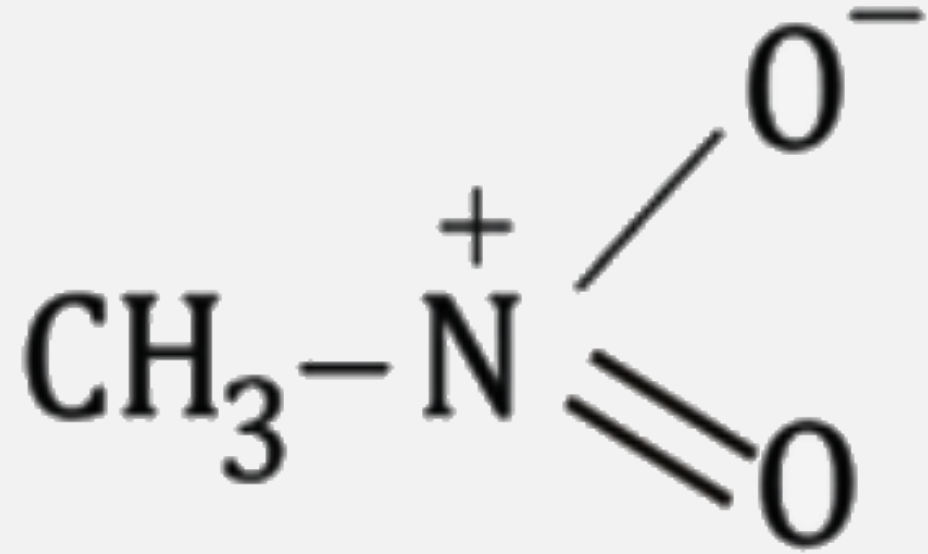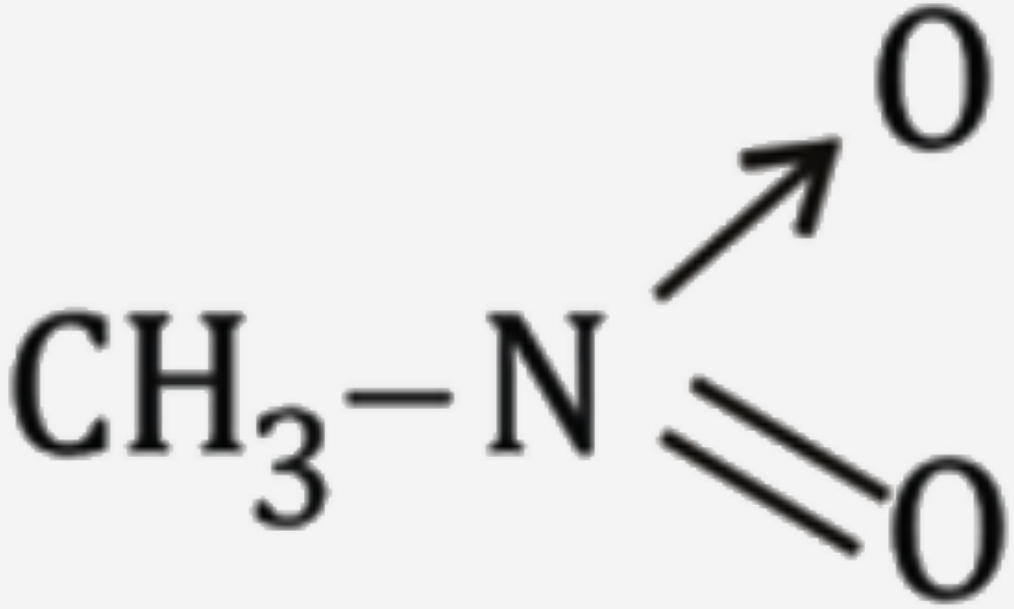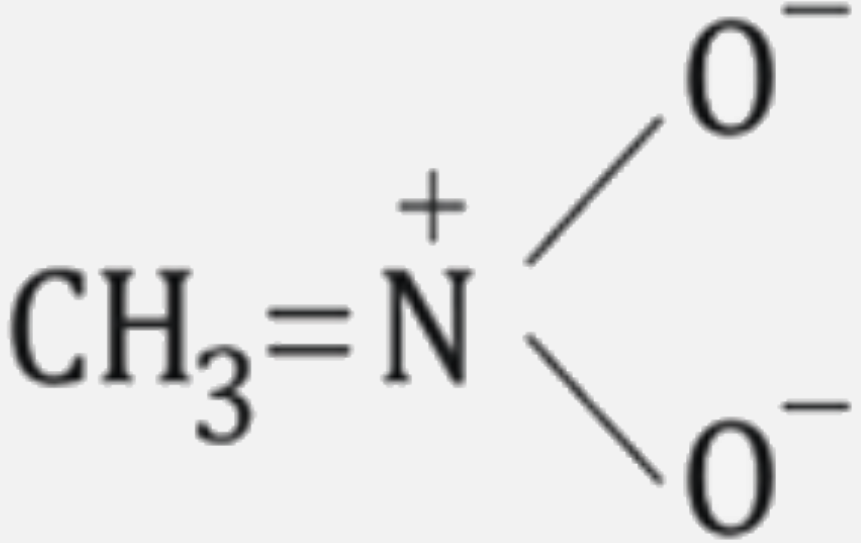A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 62-CHEMISTRY
- Which of the following is a disproportionation reaction?
Text Solution
|
- Which of the following is not a characteristic of alcohol ?
Text Solution
|
- Which of the following complexes are not correctly matched with the hy...
Text Solution
|
- Benzaldehyde can be prepared from benzene by passing vapours of ……. a...
Text Solution
|
- The above change can be effected by I. KMnO(4)//H^(+) II. [Ag(NH3)...
Text Solution
|
- An AB solid has CsCl type of structure, where A occupies corner. If th...
Text Solution
|
- One word answer is given for the following definitions, Mark the one w...
Text Solution
|
- alpha-helix is a secondary structure of proteins formed by twisting of...
Text Solution
|
- Yellow coloured aquesous solution of sodium chromate changes to orange...
Text Solution
|
- Fe^(3+) compounds are more stable than Fe^(2+) compounds because
Text Solution
|
- Which of the following is not a structure of nitromethane molecule?
Text Solution
|
- Arrange the following compounds in increasing order of their reactivit...
Text Solution
|
- What mass of hydrochloric acid is needed to decompose 50 g of limeston...
Text Solution
|
- The value of BOD of highly polluted water is
Text Solution
|
- Which of the following primary and secondary valencies are not correct...
Text Solution
|
- The structure given below is known as
Text Solution
|
- If one strand of DNA has the sequence ATGCTTGA, the sequence in the co...
Text Solution
|
- What is the lowest value of n that allows g orbitals to exist?
Text Solution
|
- The shape of water molecule, which should be tetrahedral has a bent or...
Text Solution
|
- For the reation given below, identify X in the reaction CH3C -= CCH3...
Text Solution
|