A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 67-CHEMISTRY
- The volume of atom present in a face-centred cubic unit cell of a meta...
Text Solution
|
- Which fo the following statements about zeolites is not correct ?
Text Solution
|
- A compound Z with molecular formula C(3)H(9)N reacts with C(6)H(5)SO(2...
Text Solution
|
- Identify a reagent from the following list which can easily distinguis...
Text Solution
|
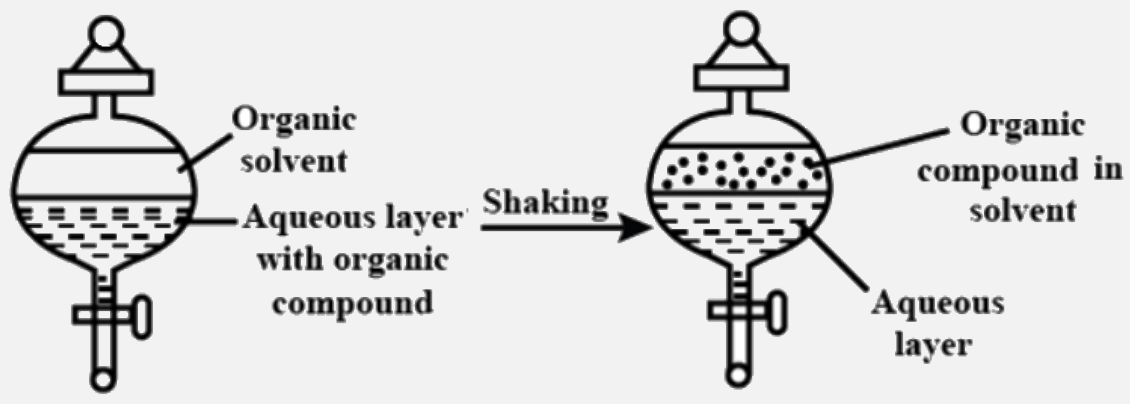
- The process of separation of an organic compound from its aqueous solu...
Text Solution
|
- Phosphorous acid on heating gives the following products: 4H(3)PO(3)...
Text Solution
|
- Why is benzene diazonium chloride not stored and is used immediately a...
Text Solution
|
- when plaster of paric comes in contact with water it sets into a hard ...
Text Solution
|
- What is the pl of glycine ? The structure and pKa values are shown bel...
Text Solution
|
- The solubility product of AgCl is 1.8xx10^(-10). Precipitation of AgCl...
Text Solution
|
- SbF(5) reacts with XeF(4) to form an adduct. The shapes of cation and ...
Text Solution
|
- Which of the following pairs of ions have the same electronic configu...
Text Solution
|
- Ph-overset(O)overset(||)C-NH2overset(POCl3)rarr(A), Product (A) is
Text Solution
|
- Which of the following is not correctly matched ?
Text Solution
|
- K(1) & K(2) for oxalic acid are 6.5 xx 10^(-2) and 6.1 xx 10^(-5) resp...
Text Solution
|
- The number of unpaired electrons in Ni (CO)(4) is
Text Solution
|
- Which is the strongest Lewis acid?
Text Solution
|
- Why partial roasting of sulphide ore is done in the metallurgy of copp...
Text Solution
|
- If hydrogen and oxygen are mixed and kept in the same vessel at room t...
Text Solution
|
- Match the column I with column II and mark the appropriate choice. {...
Text Solution
|