Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-ACIDS, BASES AND SALTS-Acids, Bases And Salts
- Which of the following are present in a dilute aqueous solution of hyd...
Text Solution
|
- Identify the correct representation of reaction occurring during chlor...
Text Solution
|
- Match the acids given in Coplumn I with their correct source given in ...
Text Solution
|
- Match the important cvhemicals given in column I with the chemical for...
Text Solution
|
- What will be the action of the following substances on litmus paper? ...
Text Solution
|
- Name the acid present in ant sting and give its chemical formula. Also...
Text Solution
|
- What happens when nitric acid is added to egg shell ?
Text Solution
|
- A student prepared solutions of (i) an acid and (ii) a base in two sep...
Text Solution
|
- How would you distinguish between baking powder and washing soda by he...
Text Solution
|
- Salt 'P', commonly used in bakery products, on heating gets con...
Text Solution
|
- In one of the industrial processes for manufacture of sodium hydroxide...
Text Solution
|
- Fill in the missing data in the given table
Text Solution
|
- What are strong and weak acids? In the following list of acids separat...
Text Solution
|
- When zinc metal is treated with a dilute solution of a strong acid, a...
Text Solution
|
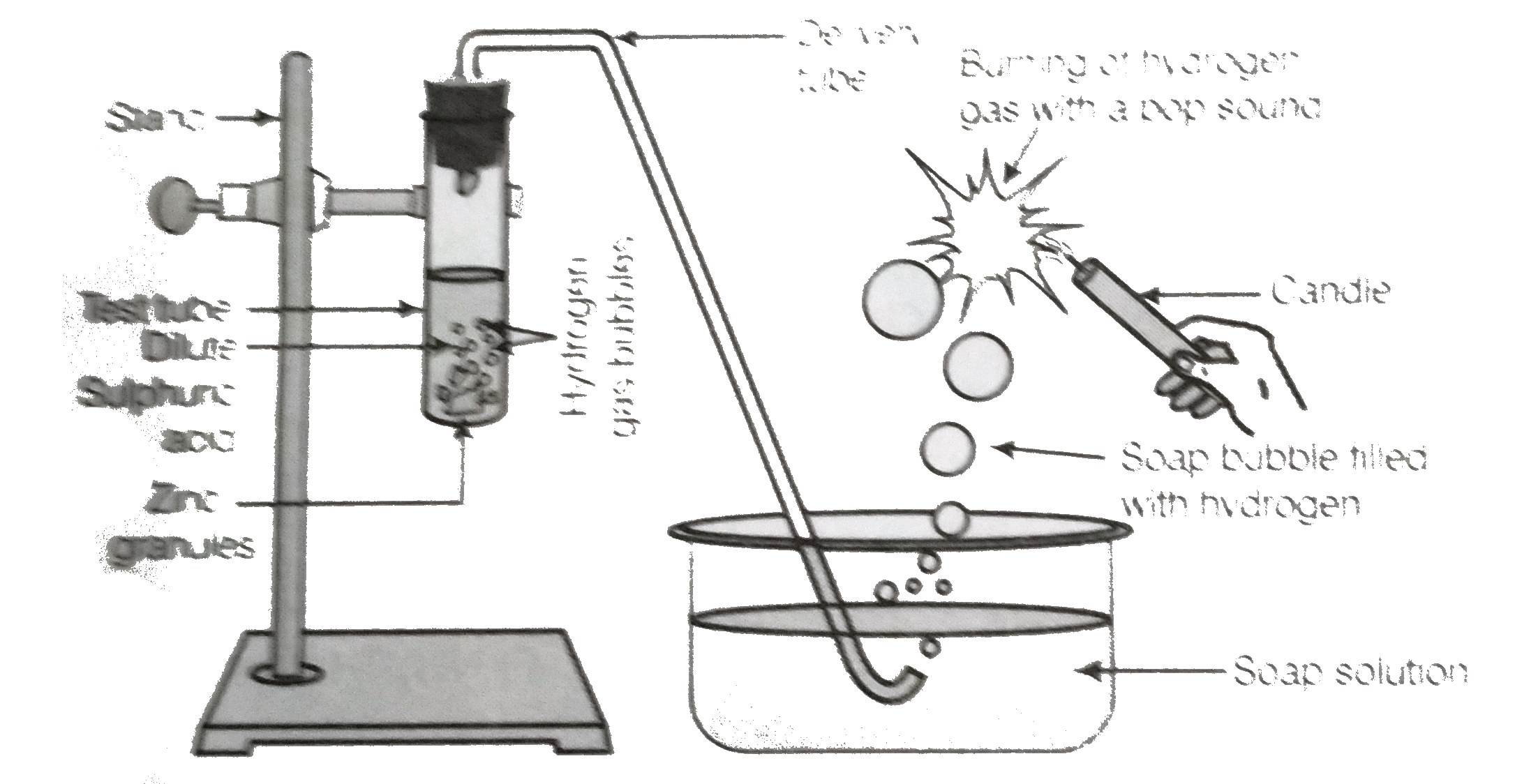
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- For making cake, baking powder is taken. If at home your mother uses b...
Text Solution
|
- A metal carbonate X on reacting with an acid gives a gas which when p...
Text Solution
|
- A dry pellet of a common base B, when kept in open absorbs moisture an...
Text Solution
|
- A sulphate salt of group 2 element of the periodic table is a white so...
Text Solution
|
- Identify the compound X on the basis of the reactions given below. Al...
Text Solution
|