Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MATTER IN OUR SURROUNDINGS
LAKHMIR SINGH & MANJIT KAUR|Exercise VERY SHORT ANSWER TYPE QUESTIONS|6 VideosMATTER IN OUR SURROUNDINGS
LAKHMIR SINGH & MANJIT KAUR|Exercise SHORT ANSWER TYPE QUESTIONS|13 VideosIS MATTER AROUND US PURE
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT BOOK PAGE 24|1 VideosMODEL TEST PAPER 1
LAKHMIR SINGH & MANJIT KAUR|Exercise SECTION B|3 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-MATTER IN OUR SURROUNDINGS -NCERT
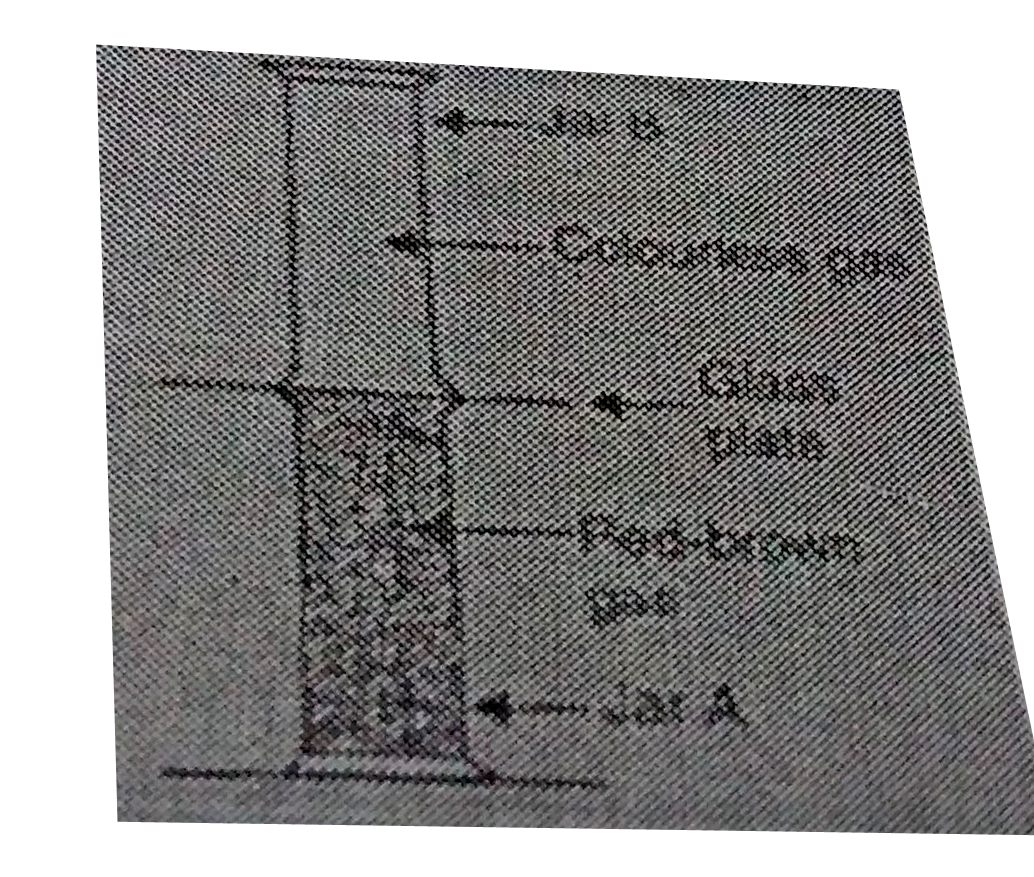
- Look at the diagram on the right side, jar A contains a red-brown gas ...
Text Solution
|
- Which of the following are matter? Chair, air,love, smell, hate, atmon...
Text Solution
|
- Give reasons for the following observation: The smell of hot sizzling ...
Text Solution
|
- A diver is able to cut through water in a swimming pool. Which propert...
Text Solution
|
- CHARACTERISTICS OF PARTICLES OF MATTER
Text Solution
|
- The mass per unit volume of a substance is called density (density = m...
Text Solution
|
- (a) Tabulate the differences in the characteristics of states of matte...
Text Solution
|
- Give reasons (a) A gas fills completely the vessel in which it is ke...
Text Solution
|
- Liquids generally have lower density as compared to solids but you mus...
Text Solution
|
- Convert the following temperatures to celsius scale : (a) 300 K (...
Text Solution
|
- What is the physical state of water at: a. 250^(@)C b. 100^(@)C ?
Text Solution
|
- For any substance, why does the temperture remain constant during the ...
Text Solution
|
- Suggest a method to liquefy atmospheric gases.
Text Solution
|
- Why does a desert cooler cool better on a hot dry day?
Text Solution
|
- How does the water kept in an earthen pot (mataka) become cool during ...
Text Solution
|
- Why does our palm feel cold when we put some acetone or petror or per...
Text Solution
|
- Why are we able to sip hot tea or milk faster from a saucer rather tha...
Text Solution
|
- What type of clothes should we wear in summer?
Text Solution
|
- Convert the following temperatures to the celsius scale. (a) 293k (...
Text Solution
|
- Convert the folowing temperature to the kelvin scale. (a) 25^(@)C (b...
Text Solution
|
- Give reason for the following observations. (a) Naphthalene balls d...
Text Solution
|