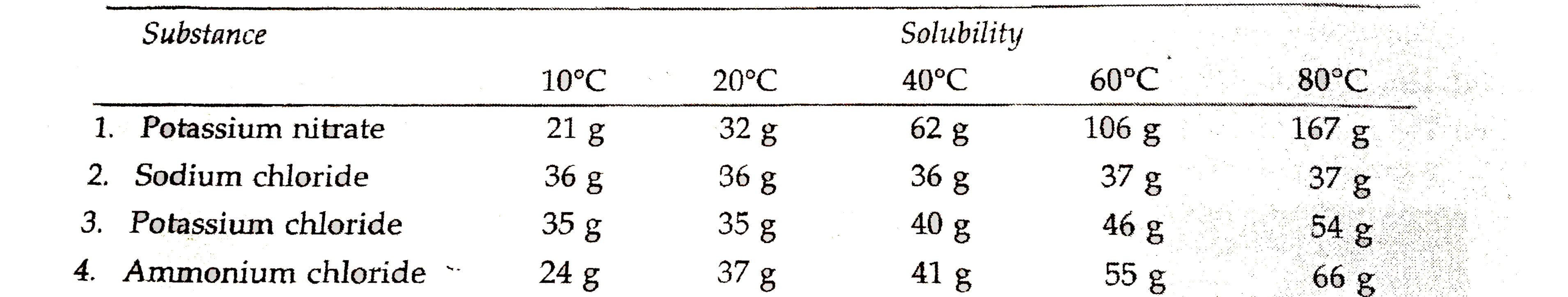Text Solution
Verified by Experts
Topper's Solved these Questions
IS MATTER AROUND US PURE
LAKHMIR SINGH & MANJIT KAUR|Exercise Exercise|247 VideosIS MATTER AROUND US PURE
LAKHMIR SINGH & MANJIT KAUR|Exercise Questions based on high order thinking skills (HOTS)|3 VideosATOMS AND MOLECULES
LAKHMIR SINGH & MANJIT KAUR|Exercise Exercise|205 VideosMATTER IN OUR SURROUNDINGS
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT|26 Videos
Similar Questions
Explore conceptually related problems