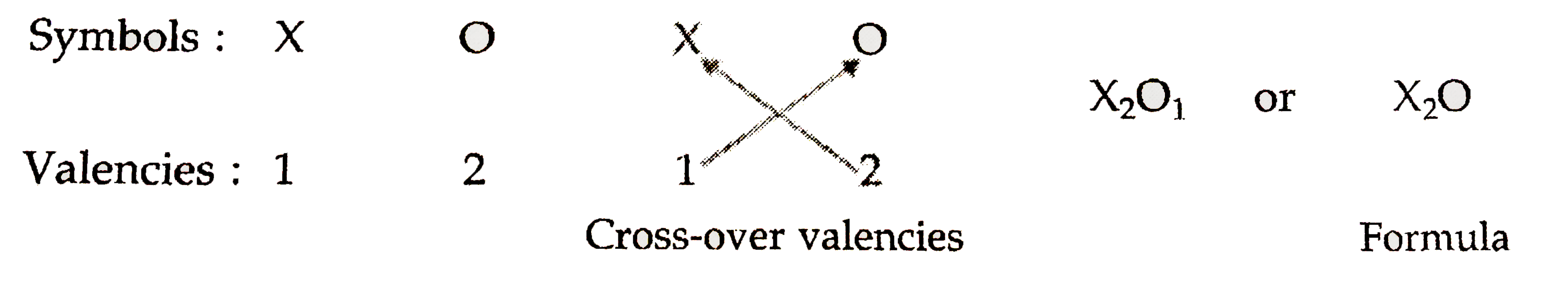Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-ATOMS AND MOLECULES-Exercise
- The valency of an element X is 1 and that of oxygen is 2. What will b...
Text Solution
|
- Write the full form of IUPAC.
Text Solution
|
- Name the scientist who gave : (a) law of conservation of mass. ...
Text Solution
|
- Name the law of chemical combination : (a) Which was given by Lavo...
Text Solution
|
- Name the scientist who gave atomic theory of matter.
Text Solution
|
- Which postulate of Dalton’s atomic theory is the result of the law o...
Text Solution
|
- Which postulate of Dalton’s atomic theory can explain the law of def...
Text Solution
|
- Which ancient India philosopher suggested that all matter is composed...
Text Solution
|
- Name any two laws of chemical combination.
Text Solution
|
- If 100 grams of pure water taken from different sources is decomposed ...
Text Solution
|
- If 100 grams of calcium carbonate (whether in the form of marble or ...
Text Solution
|
- What are the builiding blocks of matter ?
Text Solution
|
- How is the size of an atom indicated ?
Text Solution
|
- Name the unit in which the radius of an atom is usually expressed.
Text Solution
|
- Write the relation between nanometre and metre.
Text Solution
|
- The radius of an oxygen atom is 0.073 nm. What does the symbol 'nm' ...
Text Solution
|
- State whether the following statement is true or false : The symbol...
Text Solution
|
- Define 'molecular mass' of a substance.
Text Solution
|
- What is meant by saying that 'the molecular mass of oxygen is 32'...
Text Solution
|
- Fill in the following blanks with suitable words : (a) In water , t...
Text Solution
|
- (a) Name the element used as a standard for atomic mass scale. (b) W...
Text Solution
|