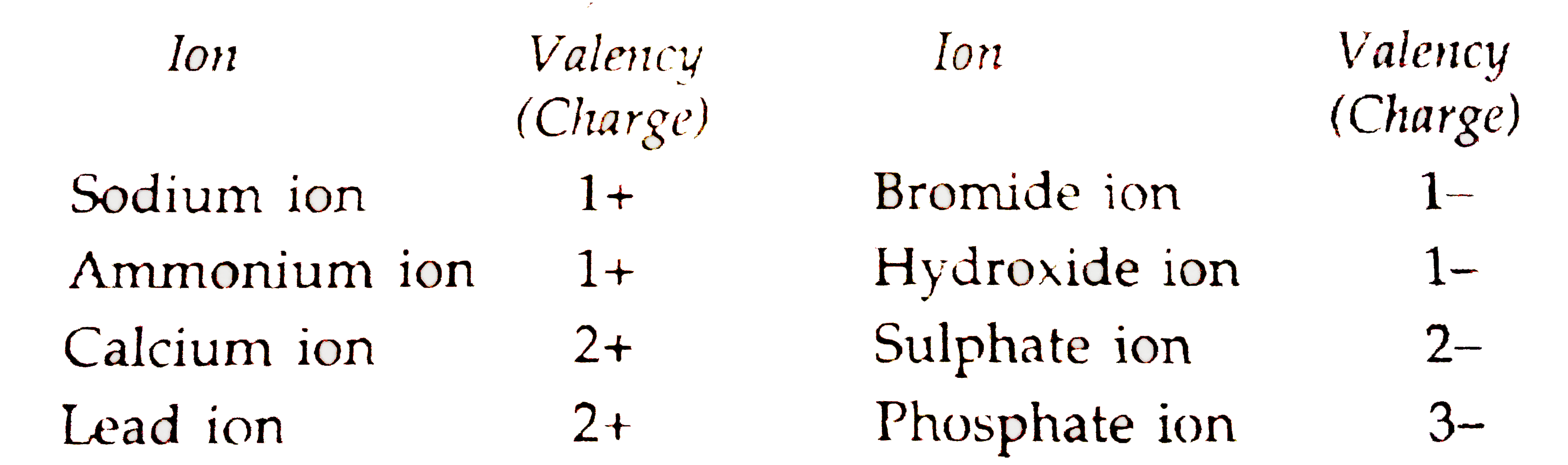Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-ATOMS AND MOLECULES-Exercise
- Give the formulae of the compounds formed from the following sets of e...
Text Solution
|
- What are ionic and molecular compounds? Give exmples.
Text Solution
|
- (a) What is an ion ? How is an ion formed ? Explain with the help of t...
Text Solution
|
- (a) What is the difference between a cation and an anion? Explain ...
Text Solution
|
- Explain the formation of (i) sodium ion, and (ii) chloride ion, from ...
Text Solution
|
- (a) Write the symbols/formulae of two simple ions and two compound io...
Text Solution
|
- (a) Define 'formula unit' of an ionic compound. What is the formul...
Text Solution
|
- The atomic number of an element X is 13. What will be the number of el...
Text Solution
|
- Which of the following represents a correct chemical formula ?
Text Solution
|
- If the number of electrons in an ion Z^(3-) is 10, the atomic number...
Text Solution
|
- The anion of an element has :
Text Solution
|
- A particle X has 17 protons, 18 neutrons and 18 electrons. This part...
Text Solution
|
- An element which can exhibit valencies of 2, 4 and 6 can be :
Text Solution
|
- The atomic number of an element E is 16. The number of electrons in i...
Text Solution
|
- The cation of an element has :
Text Solution
|
- Two elements X and Y have valencies of 5 and 3, and 3 and 2, respecti...
Text Solution
|
- The number of electrons in an ion Y^(2+) is 10. The atomic number of e...
Text Solution
|
- A particle P has 18 electrons, 20 neutrons and 19 protons. This parti...
Text Solution
|
- An ionic compound will be formed by the combination of one of the foll...
Text Solution
|
- Molecular compounds are usually formed by the combination between :
Text Solution
|