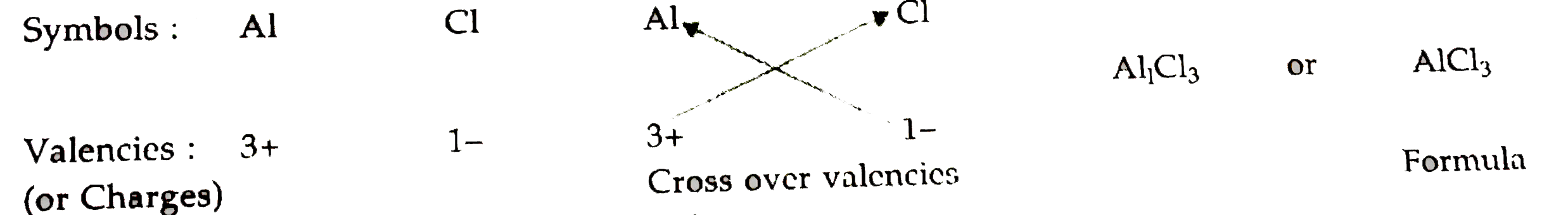Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-ATOMS AND MOLECULES-Exercise
- Define the atomic mass unit.
Text Solution
|
- Why is it not possible to see an atom with naked eyes?
Text Solution
|
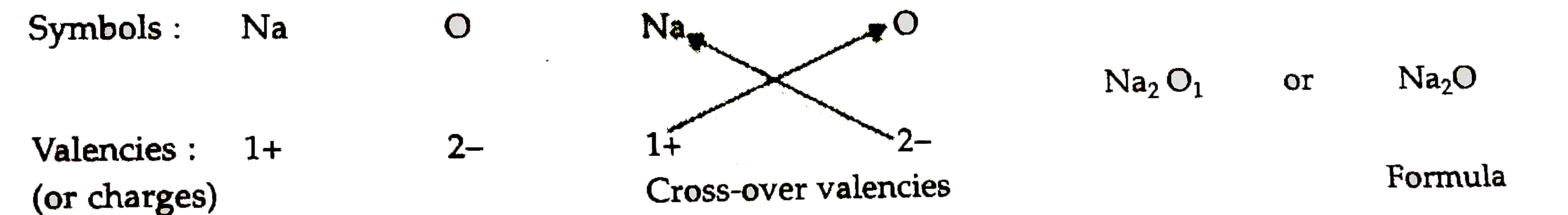
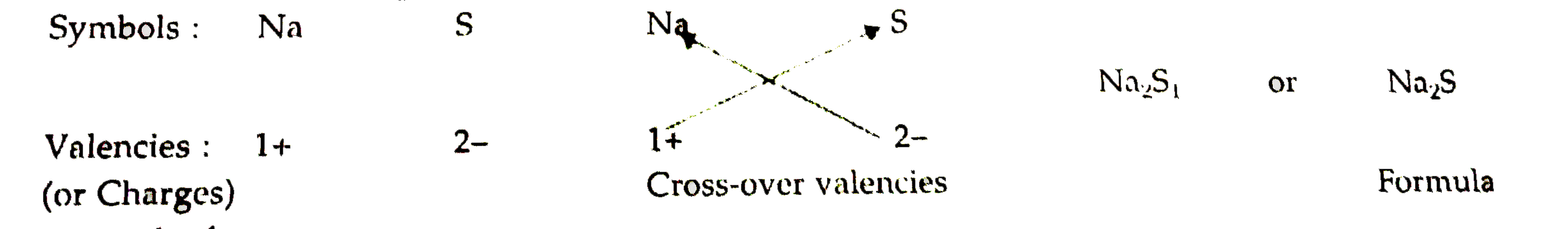
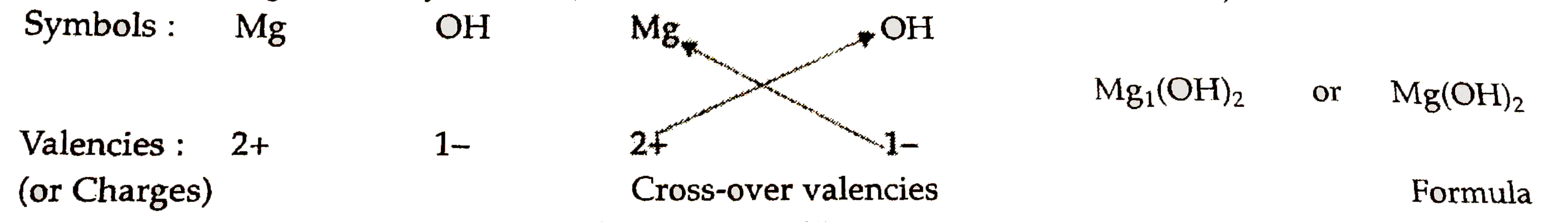
- Write down the formulae of (i) sodium oxide (ii) aluminium chlor...
Text Solution
|
- Write down the names of compounds represented by the following formula...
Text Solution
|
- What is meant by the term chemical formula?
Text Solution
|
- How many atoms are present in a (i) H(2)S molecule and (ii) PO...
Text Solution
|
- Calculate the molecular masses of H(2), O(2), Cl(2), CO(2), CH(4), C(2...
Text Solution
|
- Calculate the formula unit masses of ZnO, Na(2)O, K(2)CO(3), given ato...
Text Solution
|
- If one mole of carbon atoms weighs 12 grams, what is the mass (in gram...
Text Solution
|
- Which has more number of atoms, 100 grams of sodium or 100 grams of ir...
Text Solution
|
- A 0.24 g sample of compound of oxygen and boron was found by analysis ...
Text Solution
|
- When 3.0 g of carbon is burnt in 8.0 g oxygen, 11.0 g of carbon dioxid...
Text Solution
|
- What are polyatomic ions? Give examples.
Text Solution
|
- Write the chemical formulae of the following. (a) Magnesium chlorid...
Text Solution
|
- Give the names of the elements present in the following compounds. ...
Text Solution
|
- Calculate the molar mass of the following substances. (a) Ethyne, ...
Text Solution
|
- What is the mass of : (a) 1 mole of nitrogen atoms ? (b) 4 moles o...
Text Solution
|
- Convert into moles : (a) 12 g of oxygen gas (b) 20 g of water ...
Text Solution
|
- What is the mass of : (a) 0.2 mole of oxygen atoms? (b) 0.5 mole...
Text Solution
|
- Calculate the number of molecules of sulphur (S(8)) present in 16 g of...
Text Solution
|