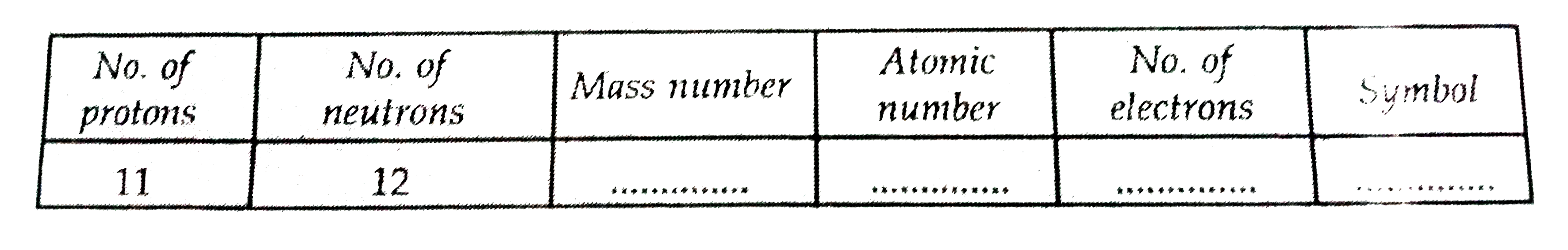Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Very Short Answer|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT Book, Page 50|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Value Based Questions|17 VideosMODEL TEST PAPER 5
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|2 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-STRUCTURE OF ATOM-Exercise
- State the location of electrons, protons, protons and neutrons in an a...
Text Solution
|
- Fill in the following blanks :
Text Solution
|
- Fill in the following blanks in respect of an atom of an element :
Text Solution
|
- (a) Describe Thomson's model of the atom. Which subatomic particle was...
Text Solution
|
- (a) Describe the Rutherford's model of an atom. State one drawback of ...
Text Solution
|
- (a) Describe Bohr's model of the atom. How did Neils Bohr explain the ...
Text Solution
|
- (a) What is mean by (i) atomic number, and (ii) mass number, of an ele...
Text Solution
|
- Rutherford's alpha particle scattering experiment led to the discovery...
Text Solution
|
- Which one of the following is a correct electronic configuration of so...
Text Solution
|
- Which subatomic particle is not present in an ordinary hydrogen atom ?
Text Solution
|
- The subatomic particle called electron was discovered by :
Text Solution
|
- Which of the following represents the correct electron distribution in...
Text Solution
|
- The correct electronic configuration of a chloride ion is :
Text Solution
|
- Goldstein's experiments which involved passing high voltage electricit...
Text Solution
|
- The number of electrons in the atom of an element X is 15 and the numb...
Text Solution
|
- The ion of an element has 3 positive charges. The mass number of atom ...
Text Solution
|
- The first model of an atom was given by
Text Solution
|
- Which of the following statement is always correct?
Text Solution
|
- From the symbol .(15)^(31)P, state : (i) mass number of phosphorus,...
Text Solution
|
- The atom of an element X has 7 electrons in its M shell (a) Write th...
Text Solution
|