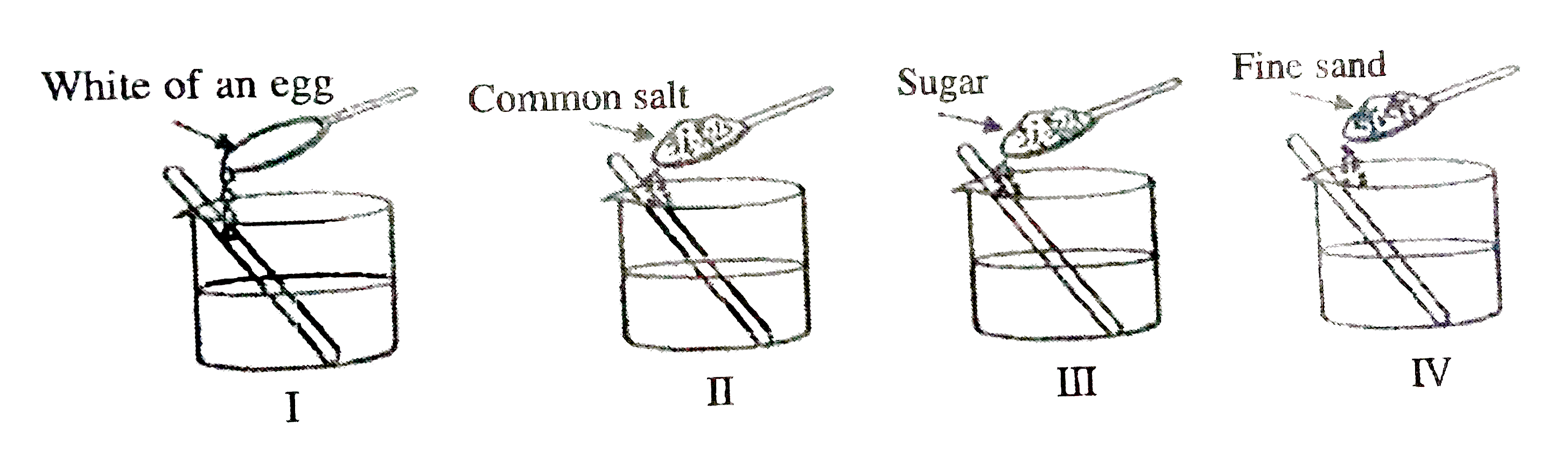A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Very Short Answer|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT Book, Page 50|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Value Based Questions|17 VideosMODEL TEST PAPER 5
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|2 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-STRUCTURE OF ATOM-Exercise
- The composition of two atomic particles is given below : {:(,"X","Y"...
Text Solution
|
- Four students (A),(B),(C) and (D) observed the colour and solubility o...
Text Solution
|
- The white of an egg, common salt, sugar and fine sand are added to wat...
Text Solution
|
- The correct procedure of heating iron-sulphur mixture to prepare iron ...
Text Solution
|
- A student while heating solid lead nitrate taken in a test tube would ...
Text Solution
|
- The following precautions were listed for the experiment on determinat...
Text Solution
|
- The correct procedure for preparing a colloidal solution of egg albumi...
Text Solution
|
- Four students (A), (B), (C) and (D) independently observed the evapora...
Text Solution
|
- A student takes a mixture of sand and ammonium chloride in a china dis...
Text Solution
|
- A student takes some water in a beaker and heats it over a flame for d...
Text Solution
|
- Which of the following is the correct set of apparatus to separate com...
Text Solution
|
- A student added the following substances to water kept in four separat...
Text Solution
|
- In an experiment, carbon disulphide was added to a test-tube containin...
Text Solution
|
- The colour of insoluble product formed sodium sulphate and barium chlo...
Text Solution
|
- A student, by mistake, mixed sulphur powder with iron filings. The fol...
Text Solution
|
- A student has done the labelling for the experimental set-up for separ...
Text Solution
|
- A teacher gave an impure sample of alum containing fine sand as impuri...
Text Solution
|
- The colour of residue left behind on heating lead nitrate when it is s...
Text Solution
|
- A student took some lead nitrate compound in a boiling tube and heated...
Text Solution
|
- When a student heated a colourless solid compound, then brown fumes of...
Text Solution
|