A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Very Short Answer|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT Book, Page 50|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Value Based Questions|17 VideosMODEL TEST PAPER 5
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|2 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-STRUCTURE OF ATOM-Exercise
- A student, by mistake, mixed sulphur powder with iron filings. The fol...
Text Solution
|
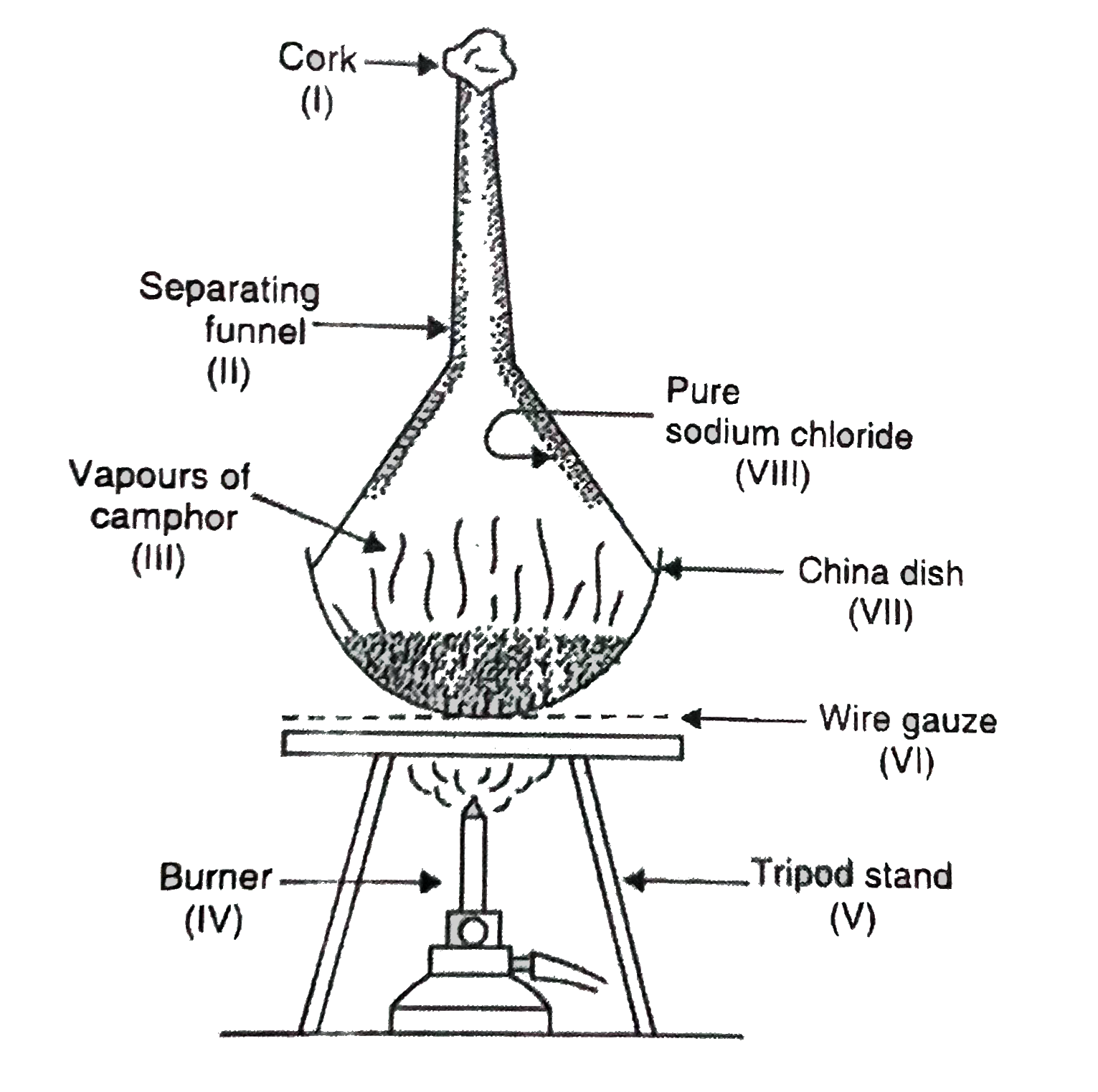
- A student has done the labelling for the experimental set-up for separ...
Text Solution
|
- A teacher gave an impure sample of alum containing fine sand as impuri...
Text Solution
|
- The colour of residue left behind on heating lead nitrate when it is s...
Text Solution
|
- A student took some lead nitrate compound in a boiling tube and heated...
Text Solution
|
- When a student heated a colourless solid compound, then brown fumes of...
Text Solution
|
- When dilute sulphuric acid is added to granulated zinc placed in a tes...
Text Solution
|
- What is the correct order of the methods you would apply to separate t...
Text Solution
|
- Four students took separately the mixture of sand, common salt and amm...
Text Solution
|
- When zinc metal reacts with dilute sulphuric acid, a gas is evolved. W...
Text Solution
|
- The reaction between iron and copper sulphate solution represents whic...
Text Solution
|
- When a burning magnesium ribbon is introduced in a gas jar containing ...
Text Solution
|
- A student heats calculated amounts of iron filings and sulphur powder ...
Text Solution
|
- Which of the following mixture can be separated completely by the proc...
Text Solution
|
- Four set ups as given here were arranged to identify the gas evolved w...
Text Solution
|
- An iron nail was dropped into copper sulphate solution. After some tim...
Text Solution
|
- A student was asked by her science teacher to prepare a true solution....
Text Solution
|
- A student placed a clean iron nail in blue coloured copper sulphate so...
Text Solution
|
- When a student added a few drops of barium chloride solution to sodium...
Text Solution
|
- A student was asked to carry out a chemical reaction by placing four d...
Text Solution
|