A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Very Short Answer|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT Book, Page 50|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Value Based Questions|17 VideosMODEL TEST PAPER 5
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|2 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-STRUCTURE OF ATOM-Exercise
- A student heats calculated amounts of iron filings and sulphur powder ...
Text Solution
|
- Which of the following mixture can be separated completely by the proc...
Text Solution
|
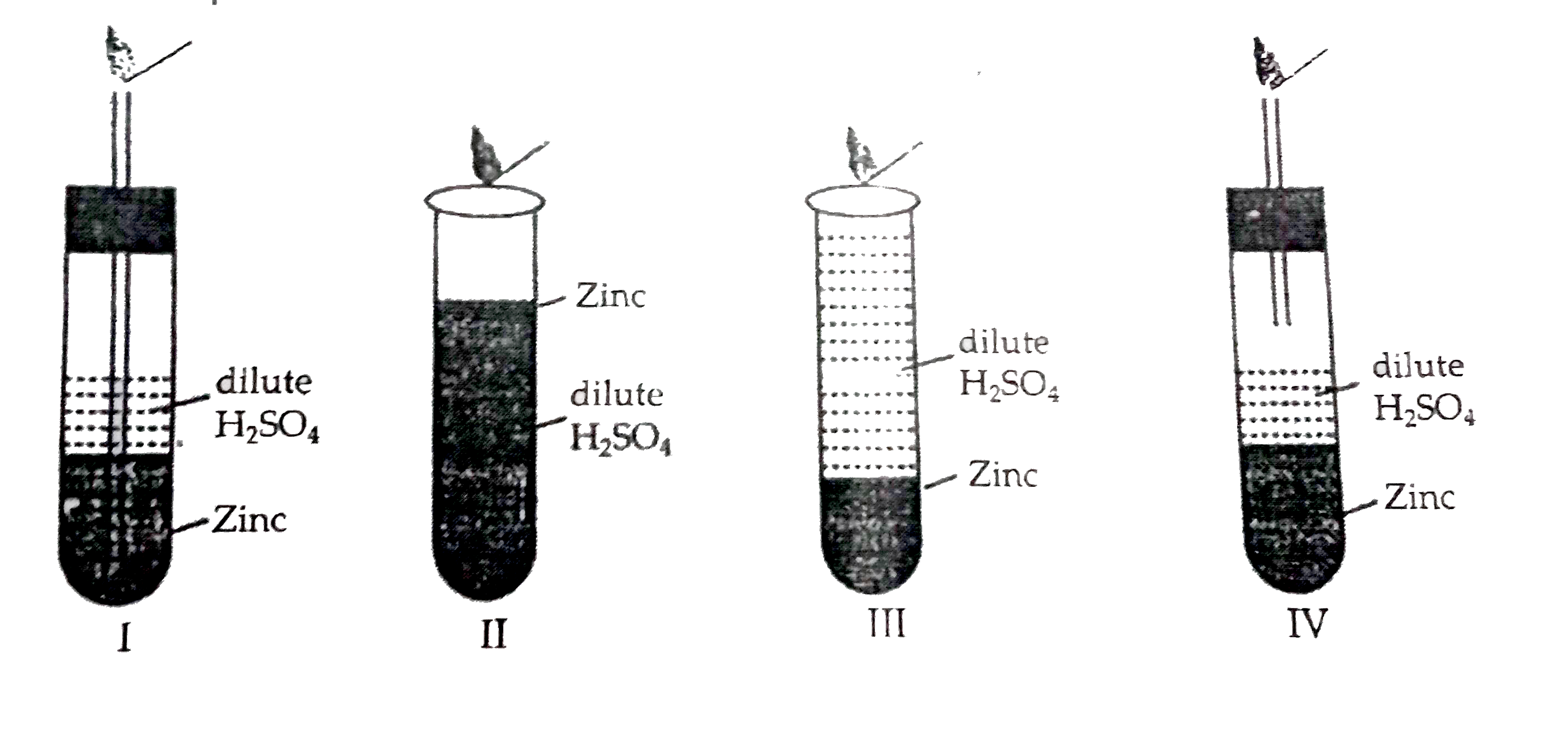
- Four set ups as given here were arranged to identify the gas evolved w...
Text Solution
|
- An iron nail was dropped into copper sulphate solution. After some tim...
Text Solution
|
- A student was asked by her science teacher to prepare a true solution....
Text Solution
|
- A student placed a clean iron nail in blue coloured copper sulphate so...
Text Solution
|
- When a student added a few drops of barium chloride solution to sodium...
Text Solution
|
- A student was asked to carry out a chemical reaction by placing four d...
Text Solution
|
- We can show that is more reactive than copper :
Text Solution
|
- A student sets up an apparatus to determine to melting point of ice. H...
Text Solution
|
- What are canal rays?
Text Solution
|
- If an atom contains one electron and one proton, will it carry any cha...
Text Solution
|
- On the basis of Thomson’s model of an atom, explain how the atom is ne...
Text Solution
|
- On the basis of Rutherford’s model of an atom, which sub- atomic parti...
Text Solution
|
- Draw a sketch of Bohr’s model of an atom with three shells.
Text Solution
|
- What do you think would be the observation if the alpha-particle scatt...
Text Solution
|
- Name the three sub-atomic particles of an atom.
Text Solution
|
- Helium atom has an atomic mass of 4u and two protons in its nucleus. H...
Text Solution
|
- If K and L shells of an atom are full, then what would be the total nu...
Text Solution
|
- How will you find the valency of chlorine, sulphur and magnesium ?
Text Solution
|