Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Very Short Answer|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT Book, Page 50|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Value Based Questions|17 VideosMODEL TEST PAPER 5
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|2 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-STRUCTURE OF ATOM-Exercise
- What are the limitations of Rutherford's model of the atom ?
Text Solution
|
- Describe Bohr's model of the atom.
Text Solution
|
- Compare all the proposed models of an atom given in this chapter.
Text Solution
|
- Summarise the rules for writing of distribution of electrons in variou...
Text Solution
|
- Define valency by taking examples of silicon and oxygen.
Text Solution
|
- Explain with examples (i) Atomic number (ii) Mass number (iii) Isotope...
Text Solution
|
- Na^(+) has completely filled K and L shells. Explain.
Text Solution
|
- Bromine occurs in nature mainly in the form of two isotopes Br(35)^(79...
Text Solution
|
- A sample of an element X contains two isotopes .(8)^(16)X and .(8)^(18...
Text Solution
|
- If Z = 3, what would be the valency of the element? Also, name the ele...
Text Solution
|
- Composition of the nuclei of two atomic species X and Y is given as un...
Text Solution
|
- For the following statements, write T for true and F for false : (a)...
Text Solution
|
- Rutherford's alpha particle scattering experiment led to the discovery...
Text Solution
|
- Isotopes of an element have : (a) the same physical properties (b)...
Text Solution
|
- Number of valence electrons in Cl^(– ) ion are:
Text Solution
|
- Which one of the following is a correct electronic configuration of so...
Text Solution
|
- Complete the following table :
Text Solution
|
- Imran and Rohan are best friends. They study in different schools in S...
Text Solution
|
- Ravi was performing some experiements related to the laws of chemical ...
Text Solution
|
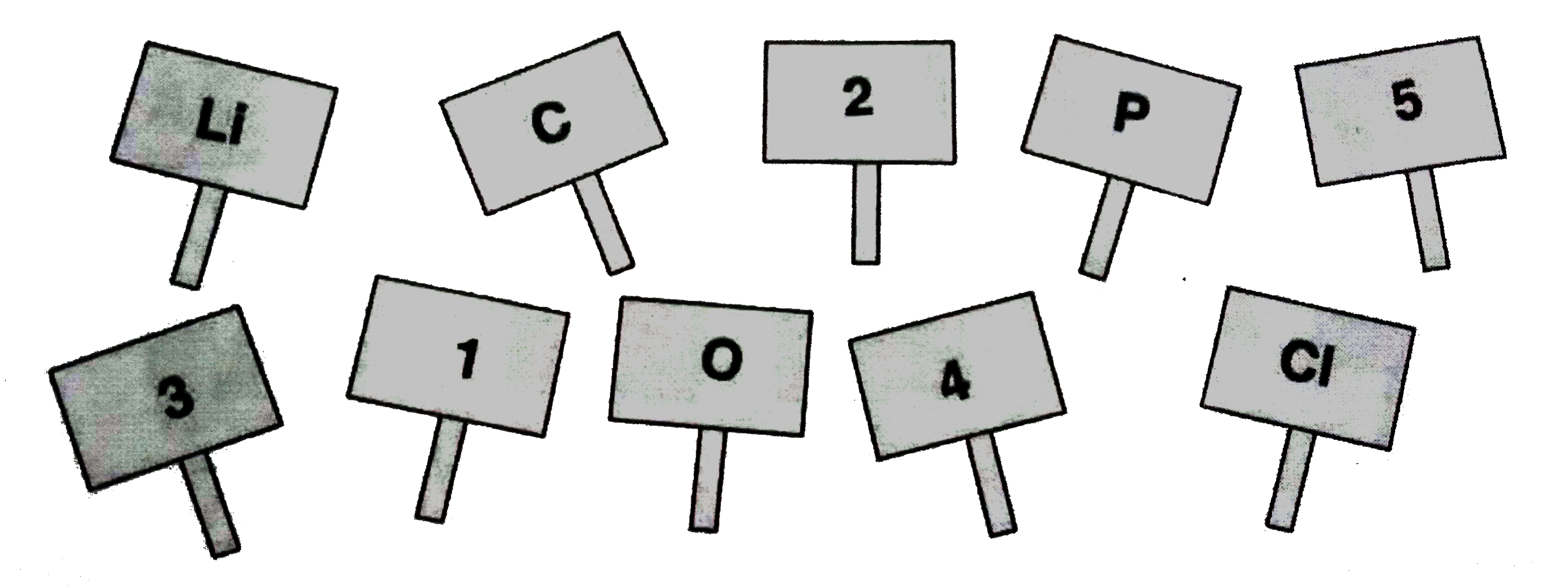
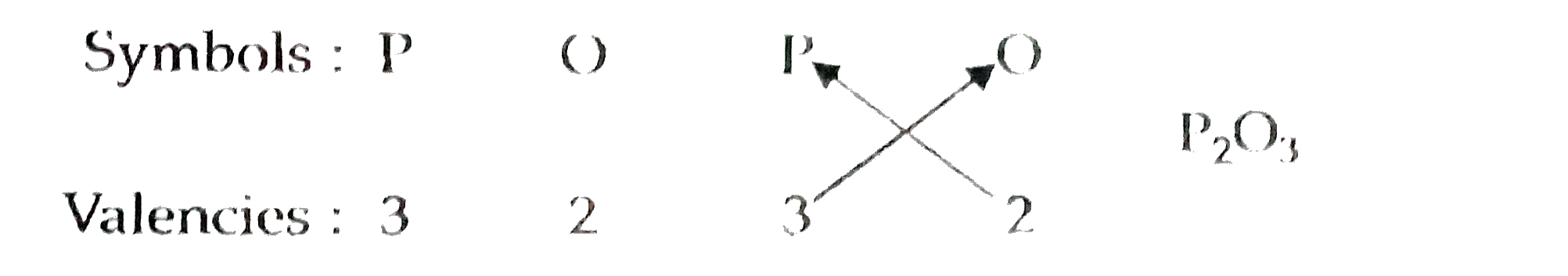
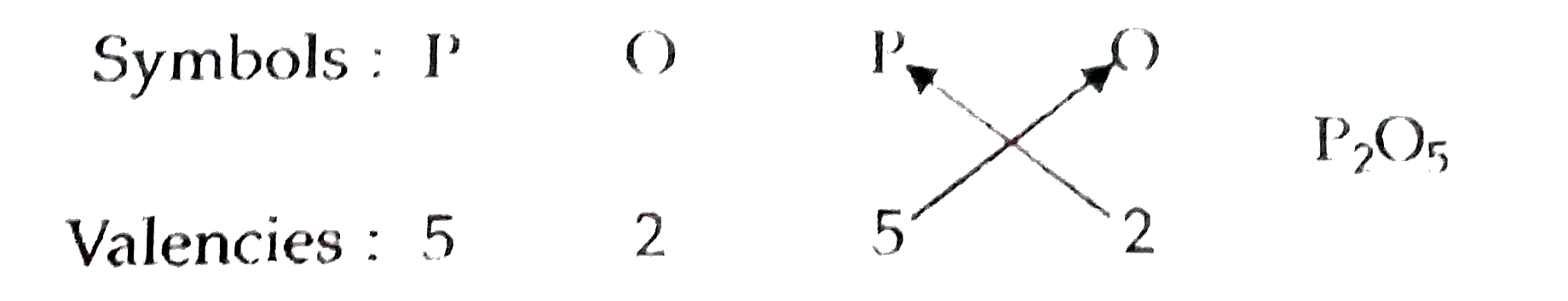
- The students of class IX have made placards showing the symbols of som...
Text Solution
|