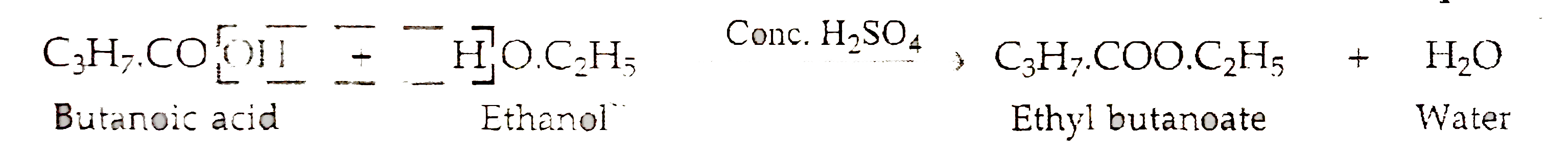Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
LAKHMIR SINGH & MANJIT KAUR|Exercise Exercise|233 VideosACID, BASES AND SALTS
LAKHMIR SINGH & MANJIT KAUR|Exercise Questions Based on High Ordre Thinking skills (HOTS)|1 VideosCHEMICAL REACTIONS AND EQUATIONS
LAKHMIR SINGH & MANJIT KAUR|Exercise QUESTIONS BASED ON HIGH ORDER THINKING SKILLS (HOTS)|1 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-CARBON AND ITS COMPOUNDS-Exercise
- The molecular formula of an ester is C(3)H(7)COOC(2)H(5). Write the mo...
Text Solution
|
- Name the element whose on eof the allotropic forms ius buckminsterfull...
Text Solution
|
- What are the two properties of carbon which lead to the huge number of...
Text Solution
|
- State whether the following statement is true or false: Diamond and ...
Text Solution
|
- Name the scientist who disporoved the vital force therory for the form...
Text Solution
|
- Name the element whose allotropic form is graphite.
Text Solution
|
- In addition to some propane and ethane , LPG cylinder contain mainly t...
Text Solution
|
- Buckminsterfullerene is a spherical molecule in which 60 carbon atoms...
Text Solution
|
- Name the black substance of pencil will the current flow through the ...
Text Solution
|
- How does graphite act as a lubricant?
Text Solution
|
- Name the hardest natureal substance known
Text Solution
|
- Which of the following molecule is called buckninsterullerence? C(90...
Text Solution
|
- Give the name an d structural formula of an alky group
Text Solution
|
- Write the elcrron dot sturcutes ofr : (i) ethane ,(ii) ethene , and (i...
Text Solution
|
- Give the IUPAC name of the following compound : C(2)H(6)
Text Solution
|
- Write the structural formula of propene.
Text Solution
|
- Write the structural formula of propyne.
Text Solution
|
- Write the structural formula of butane.
Text Solution
|
- What do you call the compounds having the same molecualr formula but d...
Text Solution
|
- Wirte the names of any two isomers represented by the moleucular formu...
Text Solution
|
- Write down (i) structural formula, and (ii) elecron dot formula, of an...
Text Solution
|