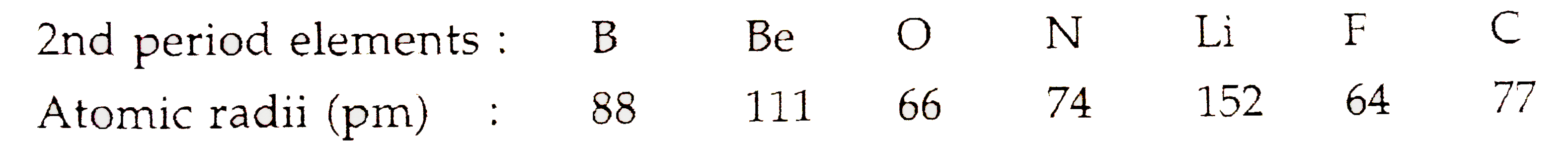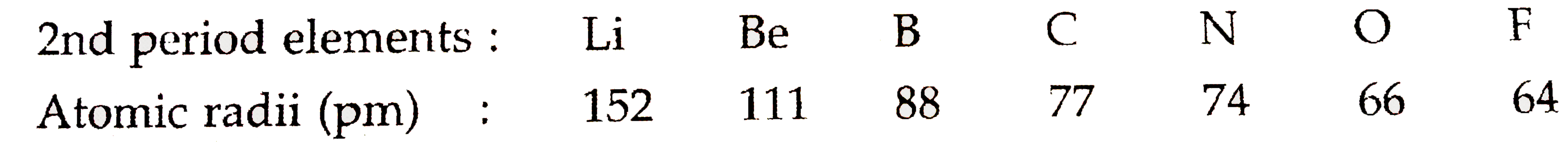Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise Exercise|215 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise SHORT ANSWER TYPE|8 VideosMETALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Short Answer Type Qustions|1 VideosTEST PAPER 1
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|4 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-PERIODIC CLASSIFICATION OF ELEMENTS-VERY SHORT ANSWER TYPE
- The atomic radii of the elements of second period are given below: ...
Text Solution
|
- (a) How does the chemical reactiviyt of alkali metals vary on going d...
Text Solution
|
- What is the major chracteristic of the first elements in the periods o...
Text Solution
|
- How do the atomic radii of elements change as we go from left to right...
Text Solution
|
- What happens to the metallic character of the elements as we go down ...
Text Solution
|
- How does the number of valence electrons vary on moving from left to r...
Text Solution
|
- How does the valency of elements change on moving from left to right i...
Text Solution
|