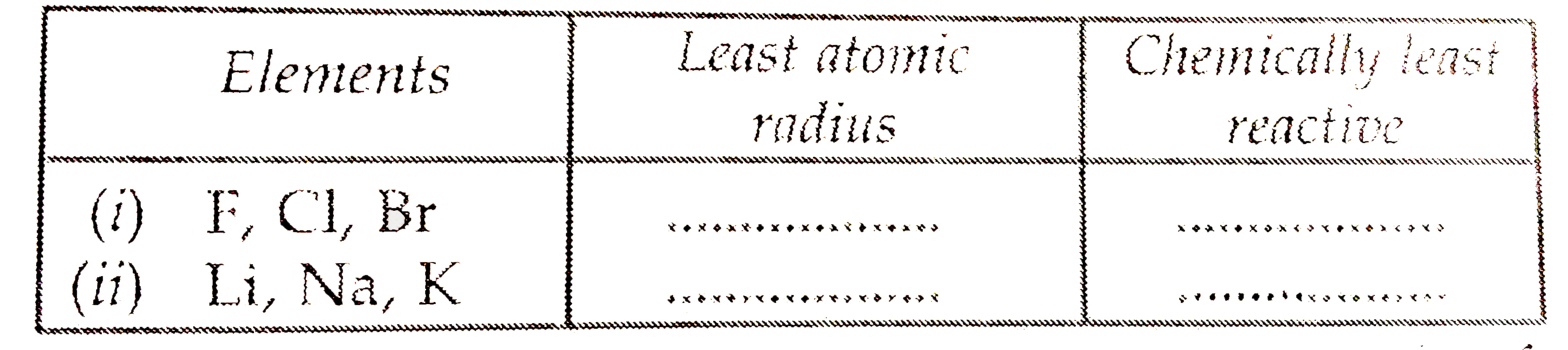Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise SHORT ANSWER TYPE|8 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise LONG ANSWER TYPE|3 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise VERY SHORT ANSWER TYPE|6 VideosMETALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Short Answer Type Qustions|1 VideosTEST PAPER 1
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|4 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise
- (a) Why does the side of the atoms progressively become smaller when w...
Text Solution
|
- (a) In the modern Periodic Table, why does cobalt with higher atomic m...
Text Solution
|
- (a) Explain why the first period of the modern periodic table has only...
Text Solution
|
- (a) What is a group in the periodic table in which part of a group wou...
Text Solution
|
- Which of the following statements is not a correct statement about the...
Text Solution
|
- The electronic configuration of the atom of an element X is 2,8,4. In ...
Text Solution
|
- The atomic number of an element is 20. In modern periodic table, this ...
Text Solution
|
- Five elements A, B, C, D and E have atomic numbers of 2, 3, 7, 10 and ...
Text Solution
|
- The elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 ...
Text Solution
|
- Which of the following elements would lose an electron easily ?
Text Solution
|
- Which of the following elements does not lose an electron easily ?
Text Solution
|
- Where would you locate the element with electronic configuration 2,8 i...
Text Solution
|
- An element which is an essential constituent of all organic compounds ...
Text Solution
|
- Which of the following is the valence shell for the elements of second...
Text Solution
|
- The element which has the maximum number of valence electrons is:
Text Solution
|
- The correct increasing order of the atomic radii of the elements oxyge...
Text Solution
|
- The atomic numbers of the elements Na, Mg, K and Ca are 11,12,19 and 2...
Text Solution
|
- Which of the following are the correct characteristics of isotopes of ...
Text Solution
|
- The correct formula of the oxide of Eka-aluminium element predicted by...
Text Solution
|
- The element which can form an acidic oxide should be the one whose ato...
Text Solution
|