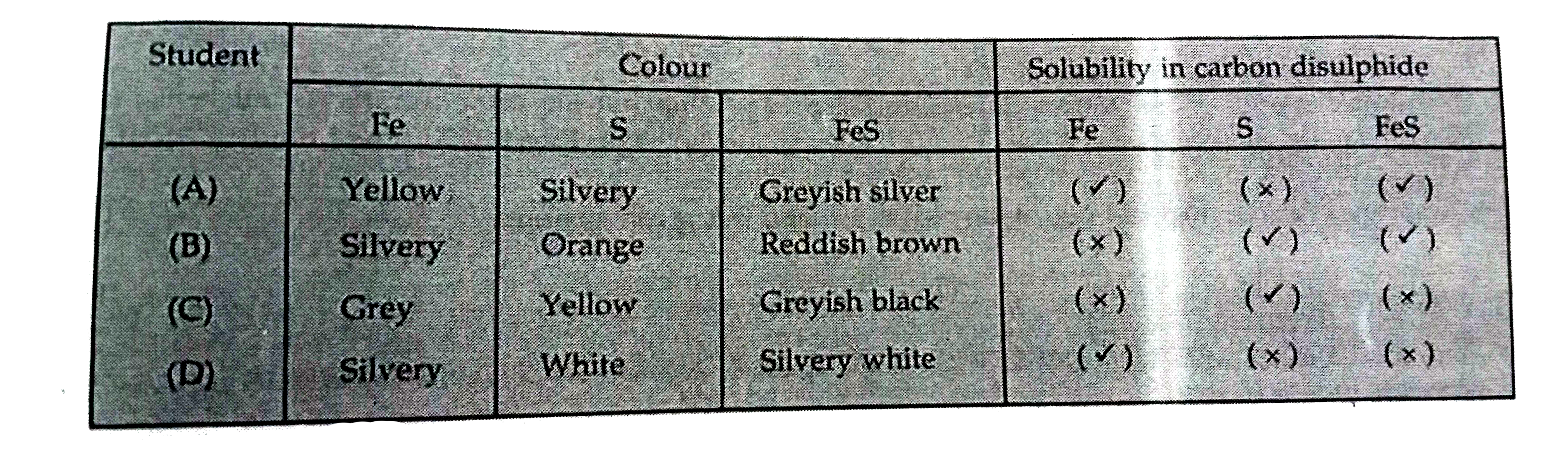
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise SHORT ANSWER TYPE|8 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise LONG ANSWER TYPE|3 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise VERY SHORT ANSWER TYPE|6 VideosMETALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Short Answer Type Qustions|1 VideosTEST PAPER 1
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|4 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise
- Four students observed the colour and odour of acetic acid and its rea...
Text Solution
|
- A student takes Cu, Al, Fe and Zn piees separtely in four test tubes l...
Text Solution
|
- Four students (A),(B),(C) and (D) observed the colour and solubility o...
Text Solution
|
- A student adds a few drops of the universal indicator solution to a di...
Text Solution
|
- A student strongly heats hydrated ferrous sulphate salt in a dry test-...
Text Solution
|
- A student took four test tubes containing solutions of different colou...
Text Solution
|
- A student while heating solid lead nitrate taken in a test tube would ...
Text Solution
|
- Four students added a small amount of ethanoic acid to sodium hydrogen...
Text Solution
|
- A student was given an unknown solution in a test tube. When he added ...
Text Solution
|
- A student placed a few drops of a liquid over a portiin of the red lit...
Text Solution
|
- A student was asked to carry out a chemical reaction by placing four d...
Text Solution
|
- When a student added universal indicator solution to one of the follow...
Text Solution
|
- A student prepared hydrogen chloride gas by treating sodium chloride w...
Text Solution
|
- When a student added a few drops of barium chloride solution to sodium...
Text Solution
|
- A student placed a pinch of solid sodium hydrogencarbonate on a strip ...
Text Solution
|
- A student placed a clean iron nail in blue coloured copper sulphate so...
Text Solution
|
- For students A,B,C and D were studying the effect of the solutions of ...
Text Solution
|
- Which of the following solutions having same concentration will have l...
Text Solution
|
- We can show that iron is more reactive than copper :
Text Solution
|
- The pH of a sample of hydrochloric acid is 2. The pH of this sample wh...
Text Solution
|