Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise SHORT ANSWER TYPE|8 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise LONG ANSWER TYPE|3 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise VERY SHORT ANSWER TYPE|6 VideosMETALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Short Answer Type Qustions|1 VideosTEST PAPER 1
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|4 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise
- The atom of an element has electronic configutation 2,8,7. (a) What ...
Text Solution
|
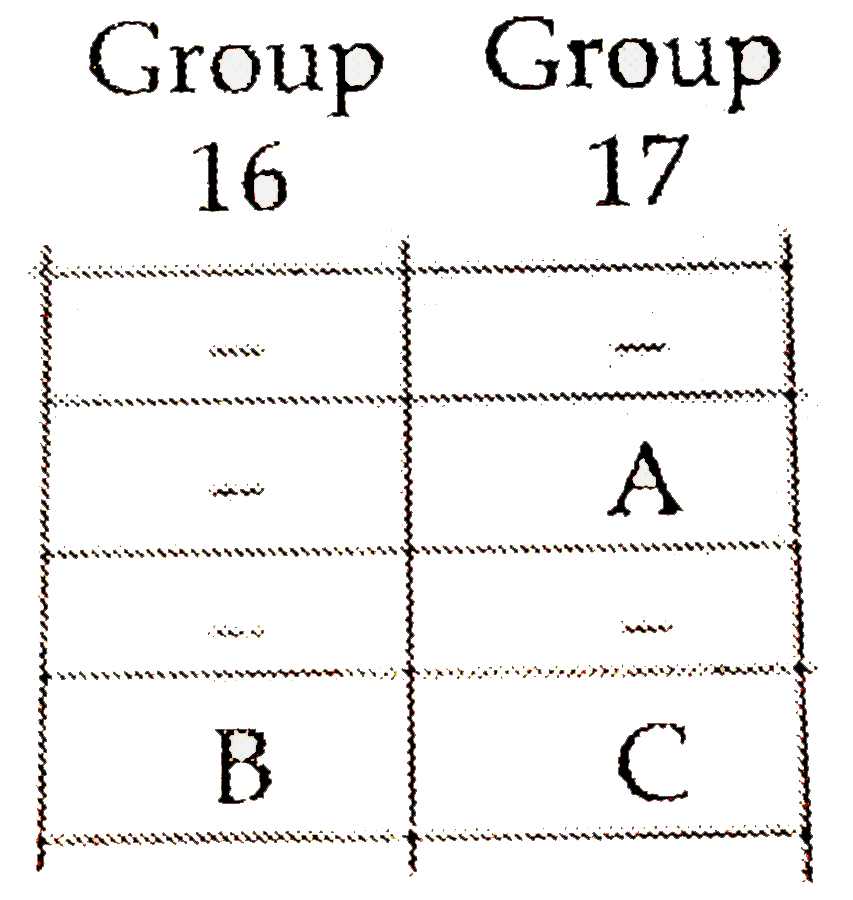
- The positions of three elements A,B and C in the periodic table are sh...
Text Solution
|
- The positions of three elements A,B and C in the periodic table are sh...
Text Solution
|
- Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to...
Text Solution
|
- How does the electronic configuration of an atom relate to its positio...
Text Solution
|
- In the modern periodic table calcium (atomic number 20) is surrounded ...
Text Solution
|
- Compare and contrast the arrangement of elements in Mendeléev’s Period...
Text Solution
|
- Rahul is a ten year old boy. He had purchased a packet of potato chips...
Text Solution
|
- Mr. Kumar runs a small garment making factory in a premises having a r...
Text Solution
|
- Raghav is a student of class X who was trying to convert the groundnut...
Text Solution
|
- Reshma is the student of class X in a city school. One day she was sit...
Text Solution
|
- Given alongside is a part of the periodic table: As we move horizont...
Text Solution
|
- How would the tendency to gain electrons change on moving from left to...
Text Solution
|
- How would the tendency to lose electrons change as we go from left to ...
Text Solution
|
- What property do all elements in the same column of the periodic table...
Text Solution
|
- What property do all the elements in the same group of the periodic ta...
Text Solution
|
- (a) What is the number of valence electrons in the atoms of first elem...
Text Solution
|
- State whether the following statement is true of fals: On going down...
Text Solution
|
- How does the valency of elements vary is going down a group of the per...
Text Solution
|
- Name the element which is in: (a) first group and third period: (b) ...
Text Solution
|