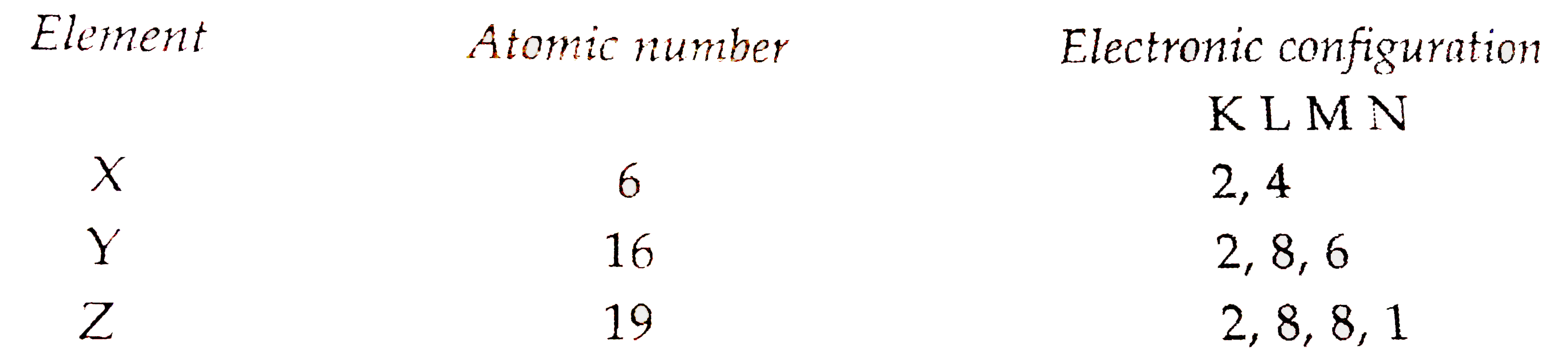Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise VERY SHORT ANSWER TYPE|6 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise MCQ_TYPE|1 VideosMETALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Short Answer Type Qustions|1 VideosTEST PAPER 1
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|4 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-PERIODIC CLASSIFICATION OF ELEMENTS-EXERCISE_TYPE
- Shailesh lies in a bigh industrial city having a large number of chem...
Text Solution
|
- Shivani is a of class X. She lives in a big house on the outskirts of ...
Text Solution
|
- Veena and Seema were coming home after attending the birthday party of...
Text Solution
|
- Bunty is a ten year old by who was playing in the park with other frie...
Text Solution
|
- Rohan and Vikram are very good friends. Rohan studies in class 9 whrea...
Text Solution
|
- Radha is a student of class X in a city school. One day Radha was doi...
Text Solution
|
- Mukesh is student of class X. He lives in a big house. There is a big...
Text Solution
|
- Arun's elder sister Rama is getting married nex month. His father and ...
Text Solution
|
- One day Anita was standing in the kitchen and talking to her mother wh...
Text Solution
|
- Abhinav studies in the nth standard in a city school. One day his scie...
Text Solution
|
- Mohan went to his ancestral village during the summer holidays t met h...
Text Solution
|
- One day Amit went to a bicycle repair shop to get broken iron part of ...
Text Solution
|
- Rahu, is studying in clas X whereas his younger brother Mohan is a stu...
Text Solution
|
- Vinod and Pramod are the best friends. Both study in class X in differ...
Text Solution
|
- Rohit's family got a wedding invitation from a relative who lives in a...
Text Solution
|
- Vineet's father has got two more rooms constructed in their existing h...
Text Solution
|
- Rohan was told that six elements A,B, C,D,E and F have atomic numbers...
Text Solution
|
- In his periodic table, Mendeleev arranged all the then known 63 elemen...
Text Solution
|
- Three are three elements X,Y and Z having atomiv numbers of 6,16 and 1...
Text Solution
|
- Devendra was told that the elements P,Q and R belong to group 2 , grou...
Text Solution
|