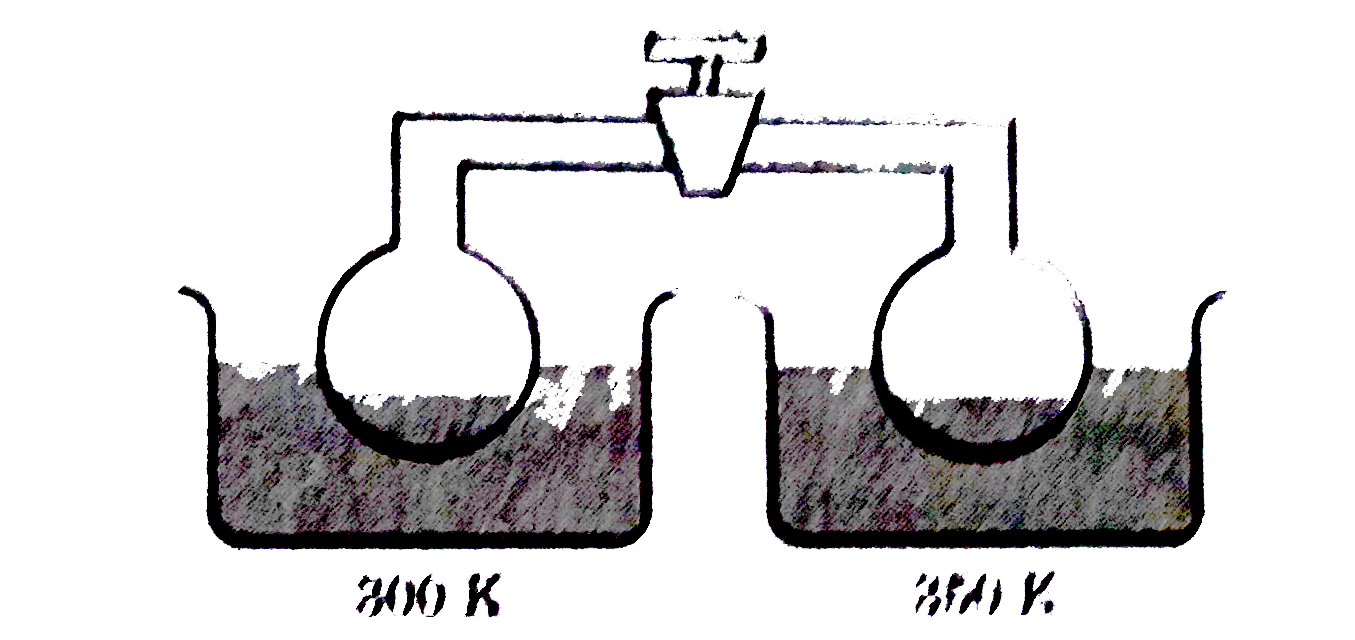Similar Questions
Explore conceptually related problems
Recommended Questions
- Two container each containing liquid water are connected as shown in d...
Text Solution
|
- Initial volume of H(2) gas saturated with water vapour is confined und...
Text Solution
|
- Two container each containing liquid water are connected as shown in d...
Text Solution
|
- At 300 K the vapour pressure of an ideal solution containing 1 mole of...
Text Solution
|
- Two container each containing liquid water are connected as shown in d...
Text Solution
|
- Calculate the vapour pressure of a solution containing 9g of glucose i...
Text Solution
|
- A solution of lactose containing 8.45 g of lactose in 100 g of water ...
Text Solution
|
- A sample of hydrogen was collected over water at 21^(@)C and 685mm Hg....
Text Solution
|
- Two containers, X and Y at 300K and 350K with water vapour pressures 2...
Text Solution
|