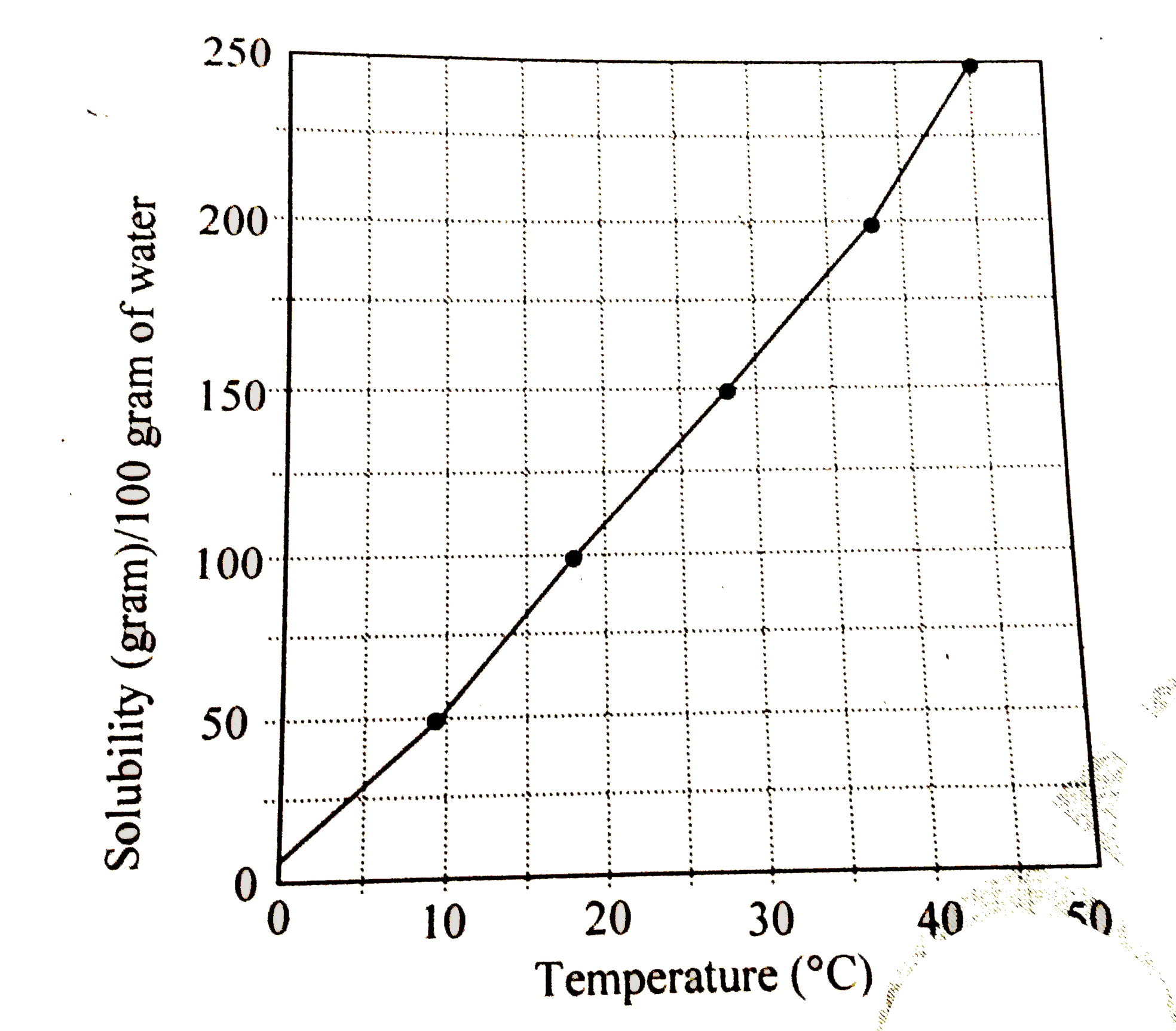
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The solubility curve of KNO(3) in water is shown below. The amou...
Text Solution
|
- The solubility of a salt in water is 40 g at 30^(@)C . The amount of w...
Text Solution
|
- A 0.01 M solution of glucose in water freezes at -0.0186^(@)C A 0.01 M...
Text Solution
|
- The solubility curve of KNO(3) in water is shown below. The amou...
Text Solution
|
- 0.06 mole of KNO(3) is added to 100cm^(3) of water at 298 K . The enth...
Text Solution
|
- 4 g of a solute are dissolved in 40 g of water to form a saturated sol...
Text Solution
|
- Solubility of KNO(3)at 313 K is 62 g. What mass of KNO(3) would be nee...
Text Solution
|
- 0.06 mole of KNO(3) is added to 100cm^(3) of water at 298K . The entha...
Text Solution
|
- एक निश्चित ताप पर 100 g जल में एक विलेय की घुलनेवाली अधिकतम मात्रा 45 ...
Text Solution
|