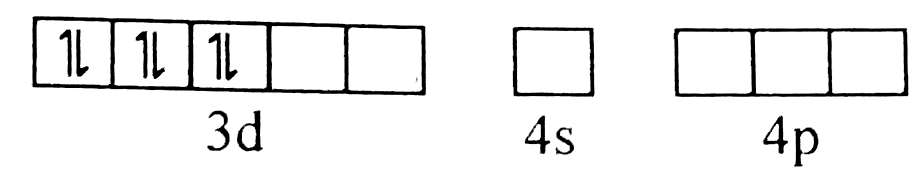
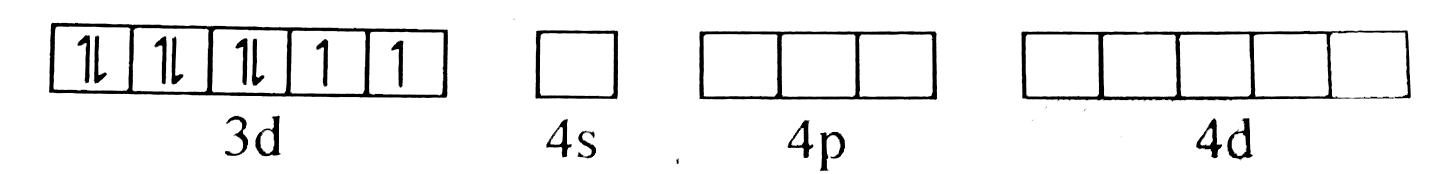
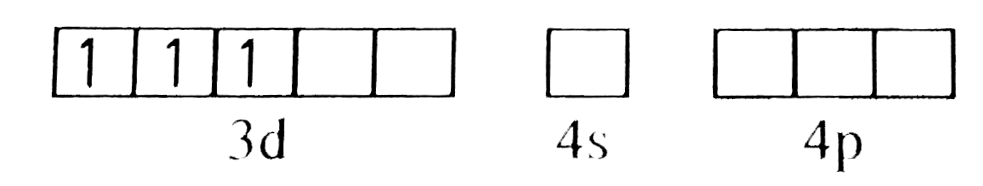
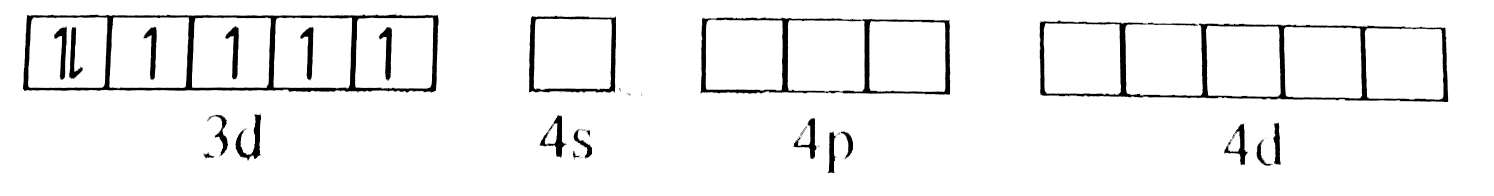
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Among (i) [Co(NH(3))(6)]Cl(3), (ii) [Ni(NH(3))(6)]Cl(2), (iii) [Cr(H(2...
Text Solution
|
- Among (i) [Co(NH(3))(6)]Cl(3), (ii) [Ni(NH(3))(6)]Cl(2), (iii) [Cr(H(2...
Text Solution
|
- The oxidation states of Cr in [Cr(H(2)O)(6)]Cl(3). [Cr(C(6)H(6))(2)]...
Text Solution
|
- The oxidation state of Cr in [Cr(H(2)O)(6)]Cl(3), [Cr(C(6)H(6))(2)], a...
Text Solution
|
- निम्नलिखित के I.U.P.A.C. नाम लिखिए - (i) [Co(NH(3))(5)]Cl(2) (ii) ...
Text Solution
|
- निम्न के IUPAC नाम लिखिए - {:((i),[Ag(NH(3))(2)]Cl,(ii),[Pt(NH(3))(2...
Text Solution
|
- Name the type of isomerism exibited by the following isomers : (1) [...
Text Solution
|
- निम्नलिखित में से धनायनिक, ऋणायनिक तथा उदासीन जटिलों को पृथक कीजिए- ...
Text Solution
|
- निम्नलिखित का IUPAC नाम लिखिए - (i) [Cr(H(2)O)(6)]Cl(3) (ii) [P...
Text Solution
|