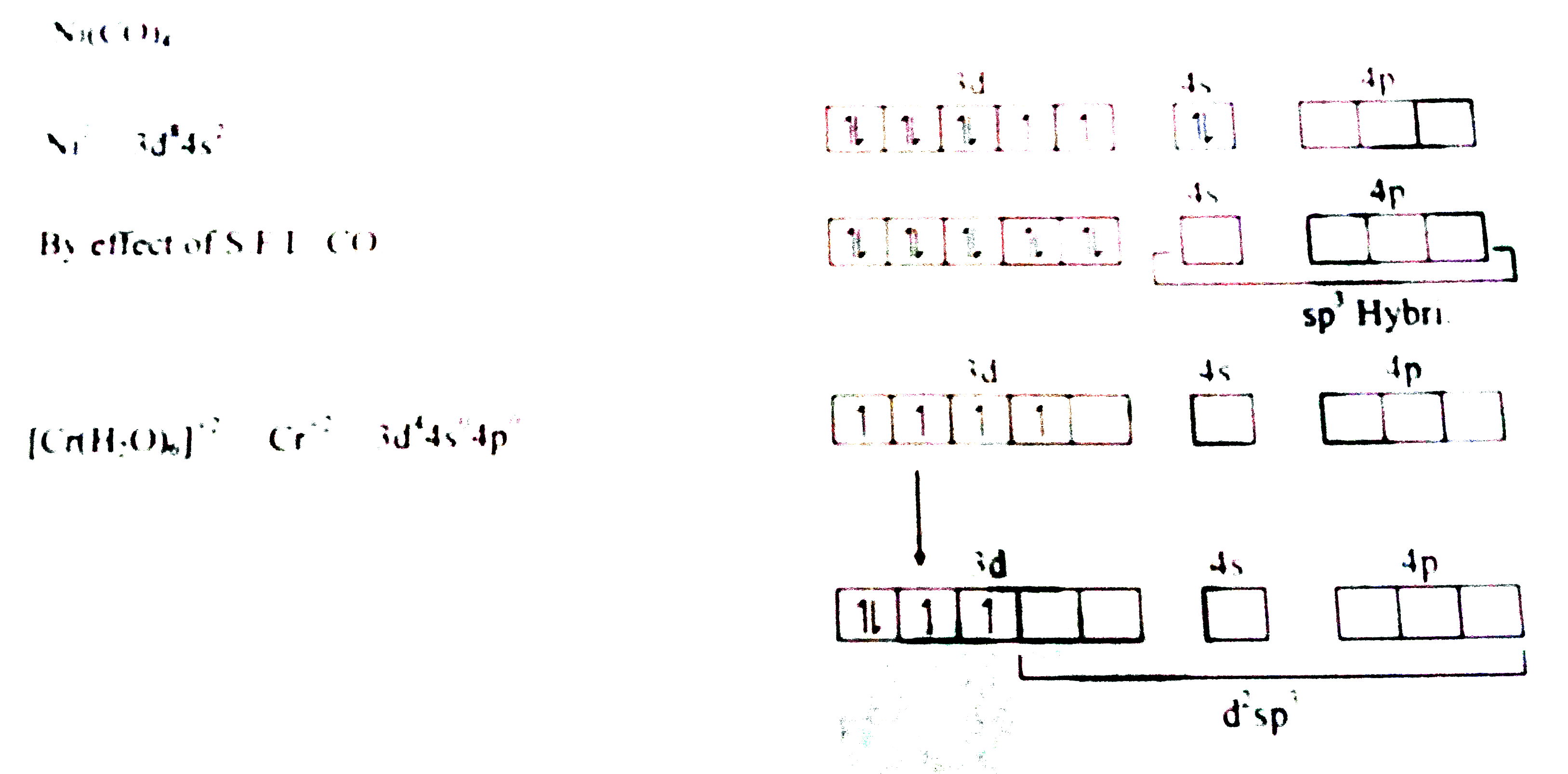A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The hybridizations of Ni(CO)(4) and Cr(H(2)O)(6)^(2+), respectively, a...
Text Solution
|
- The hybridization of [Cr(H(2)O)(6)]^(2+) is .
Text Solution
|
- The hybridizations of Ni(CO)(4) and Cr(H(2)O)(6)^(2+) , respectively, ...
Text Solution
|
- The oxidation states of Cr in [Cr(H(2)O)(6)]Cl(3). [Cr(C(6)H(6))(2)]...
Text Solution
|
- Compare the following complexes with respect to their shape , magnetic...
Text Solution
|
- Write the name, the state of hybridization, the shape and the magnetic...
Text Solution
|
- The oxidation state of Cr in [Cr(H(2)O)(6)]Cl(3), [Cr(C(6)H(6))(2)], a...
Text Solution
|
- [Ni(CO)(4)], [Ni(CN)(4)]^(2-), [NiCl(4)]^(2-) में Ni परमाणु पर संकरण क...
Text Solution
|
- Write the name, the state of hybridization, the shape and the magnetic...
Text Solution
|