A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-KVPY-PART-I CHEMISTRY
- When 1.88 g of AgBr(s) is added to a 10^(-3) M aqueous solution of KB...
Text Solution
|
- Aniline reacts with excess Br(2)//H(2)O to give the major product
Text Solution
|
- The metal with the highest oxidation state presnt in K(2)CrO(4),NbCl(...
Text Solution
|
- The number of geometrical isomers of [CrCl(2)(en)(NH(3))(2)], where en...
Text Solution
|
- The element that combines with oxygen to give an amphoteric oxide is...
Text Solution
|
- The Arrhenius plots of two reactions, I and II are shown graphically- ...
Text Solution
|
- Ni(CO)(4) is
Text Solution
|
- In the following reaction the major product X is -
Text Solution
|
- Given the structure of D-(+)- glucose as The structure of L-(-)-g...
Text Solution
|
- In a cubic close packed structure, fractional contributions of an atom...
Text Solution
|
- The equilibirum constant K(c) of the reaction, 2AhArrB+C is 0.5 at 25^...
Text Solution
|
- Major products formed in the reaction of t-butyl methyl ether with HI ...
Text Solution
|
- If the molar conductivities ("in" S cm^(2) "mol"^(-1)) of NaCl,KCl and...
Text Solution
|
- 4-Formyl benzoic acid on treatment with one equivalent of hydrazine f...
Text Solution
|
- Two elements, Xand Y, have atomic numbers 33 and 17 , rspectively. The...
Text Solution
|
- The number of moles of KMnO(4) required to oxidize one equivalent of K...
Text Solution
|
- Three successive measurements in an experiment gave the values 10.9, 1...
Text Solution
|
- The latent heat of melting of ice at 0^(@)C is 6 kJ "mol"^(-1) . The e...
Text Solution
|
- The major product of the following reaction
Text Solution
|
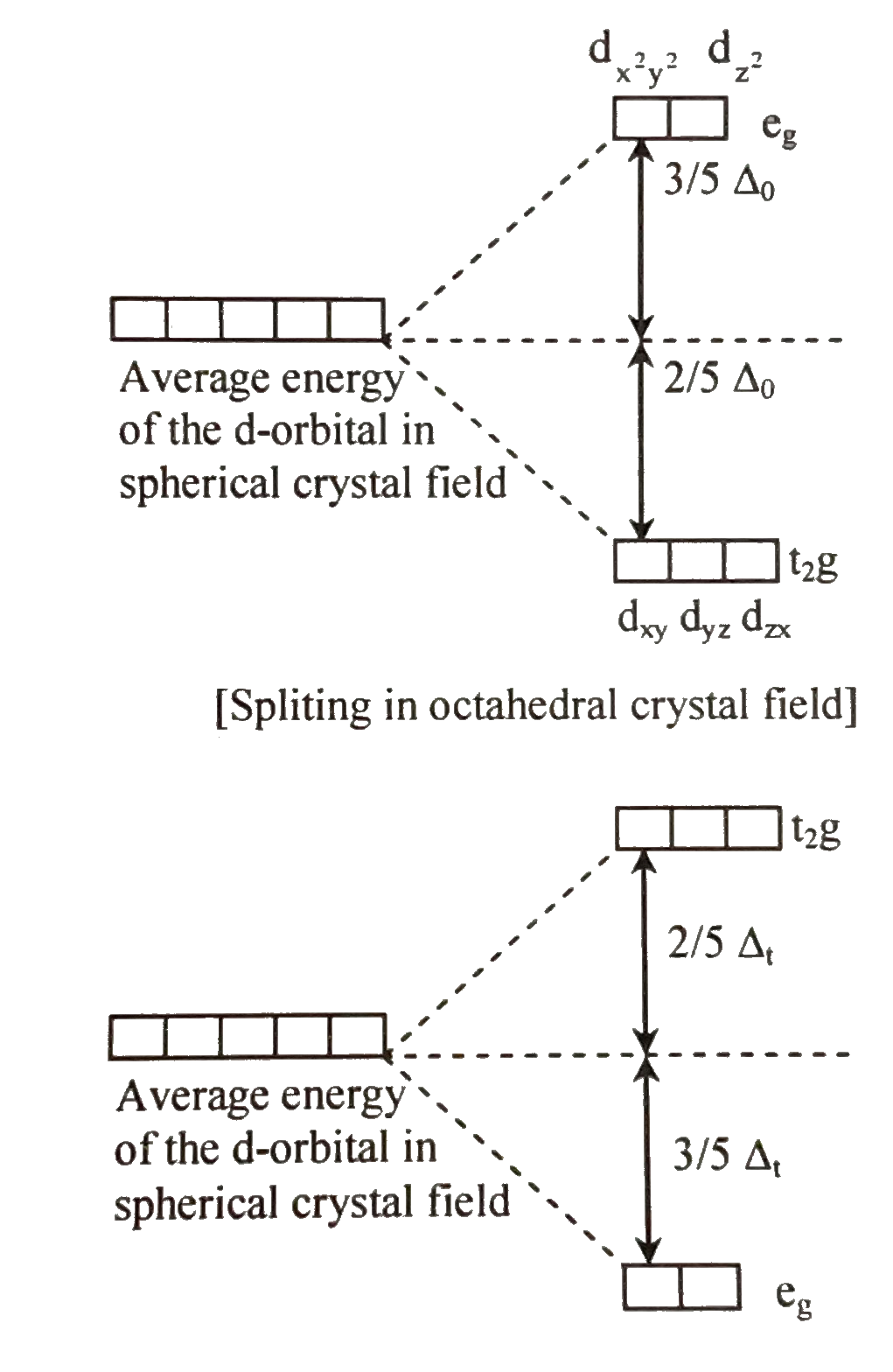
- The energies of d(xy) and d(z)^(2) orbits in octahedral and tetrahedr...
Text Solution
|