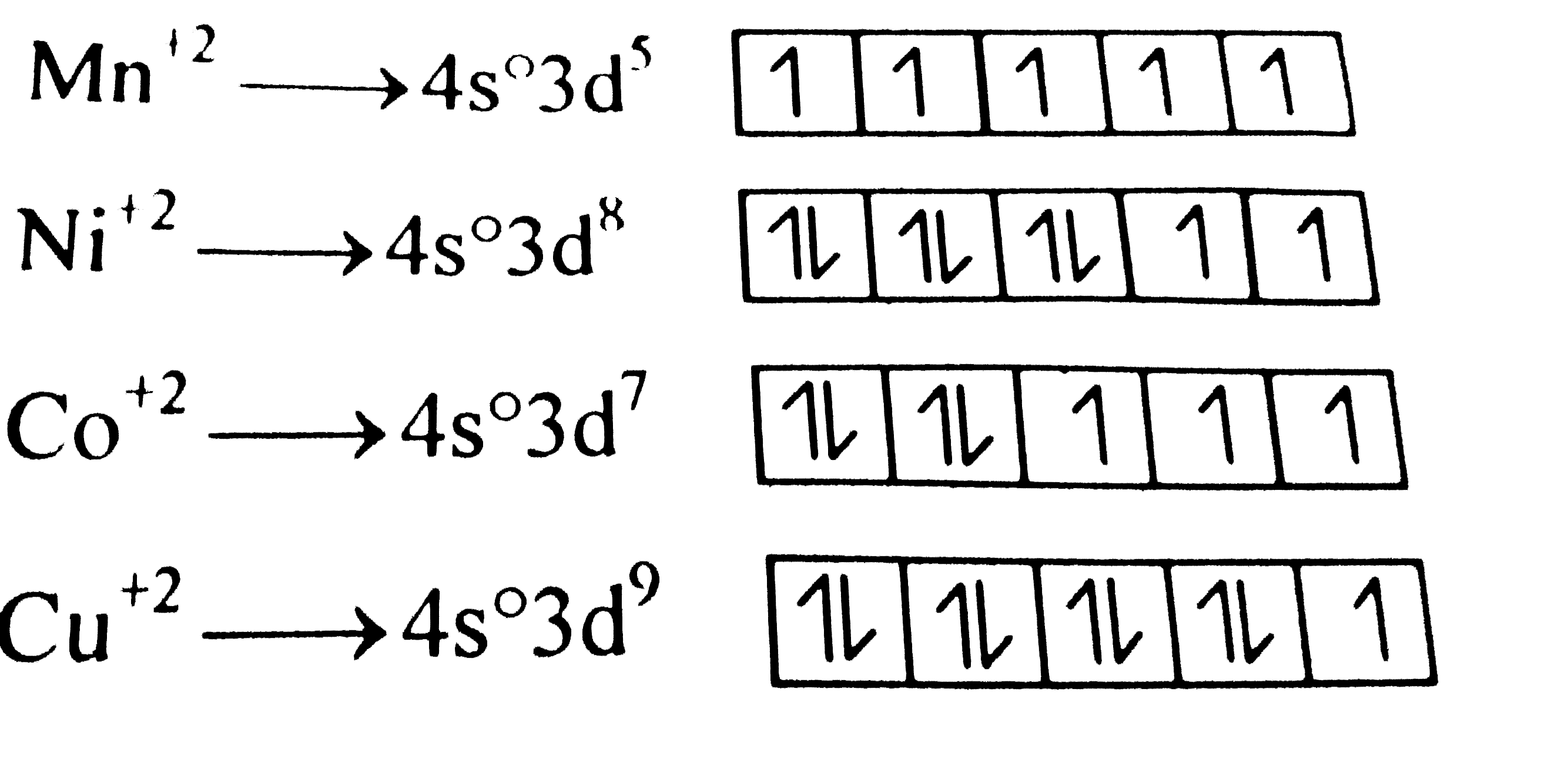
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The spin only magnetic of [ZCl(4)]^(2-) is 3.87 BM where Z is
Text Solution
|
- Assertion: The spin only magnetic moment of Sc^(3+) is 1.73 BM. Reas...
Text Solution
|
- The theoritical spin-only magnetic moment of cobalt in Hg[Co(SCN)(4)] ...
Text Solution
|
- The spin only magnetic of [ZCl(4)]^(2-) is 3.87 BM where Z is
Text Solution
|
- Which of the complexes has the magnetic moment of 3.87 BM ?
Text Solution
|
- The spin only magnetic moment of Fe^(2+) ion (in BM) is approximately
Text Solution
|
- The magnetic moment (in BM) of Zn^(2+) ion according to spin-only form...
Text Solution
|
- प्रचक्रण मात्र चुम्बकीय आघूर्ण (spin-only magnetic moment) मान 2.82 BM...
Text Solution
|
- The correct electronic configuration and spin -only magnetic moment (B...
Text Solution
|