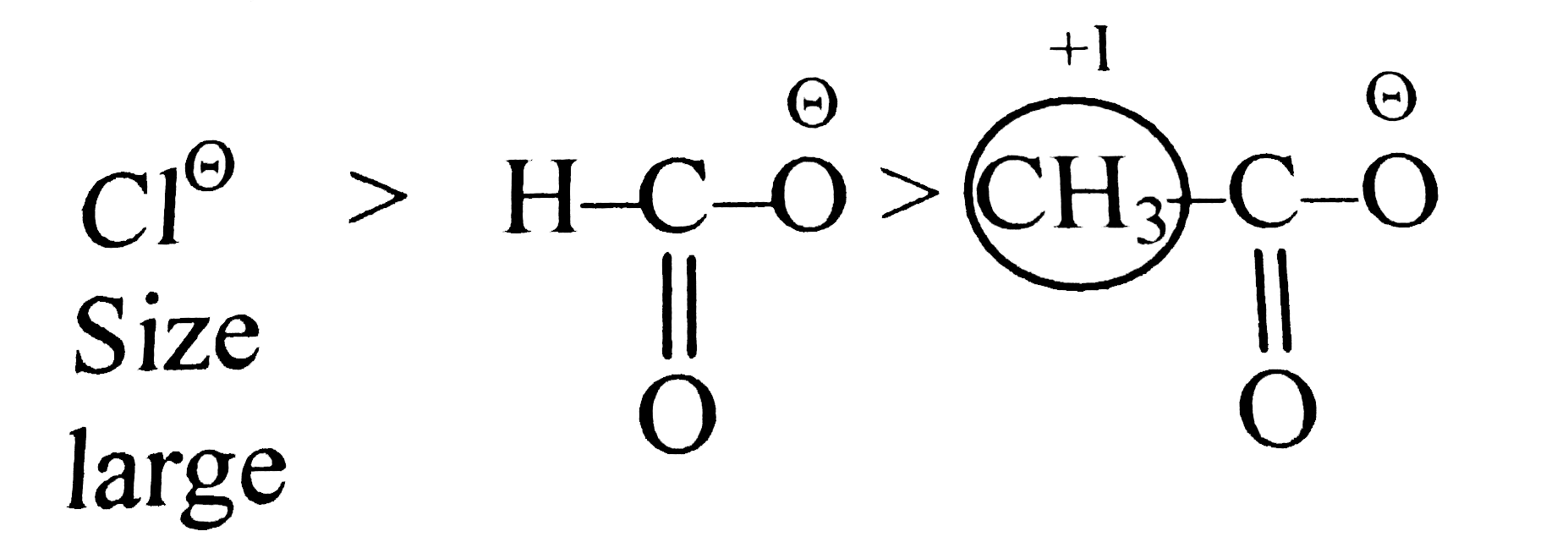
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The pH of 1N aqueous solutions HCl,CH(3)COOH and HCOOH follows the ord...
Text Solution
|
- HCOOH and CH(3)COOH solutions have equal pH. If K(1)//K(2) is 4, the r...
Text Solution
|
- Assertion (A): The pH of an aqueous solution of CH(3)COOH remains unch...
Text Solution
|
- The pH of 1N aqueous solutions HCl,CH(3)COOH and HCOOH follows the ord...
Text Solution
|
- एक जलीय विलयन में निम्नलिखित साम्य है- CH(3)COOH ltimplies CH(3)COO^...
Text Solution
|
- In CH(3)COOH "and" HCOOH,HCOOH will be .
Text Solution
|
- निम्न को बढ़ती हुई अम्लता में क्रम में लिखिए- CH(3)COOH, HCOOH, CH(3)...
Text Solution
|
- pH of an aqueous 0.1(M) CH(3)COOH solution is 2.87. state whether pH o...
Text Solution
|
- The following equilibrium exists in aqueous solution : CH(3)COOH to H^...
Text Solution
|