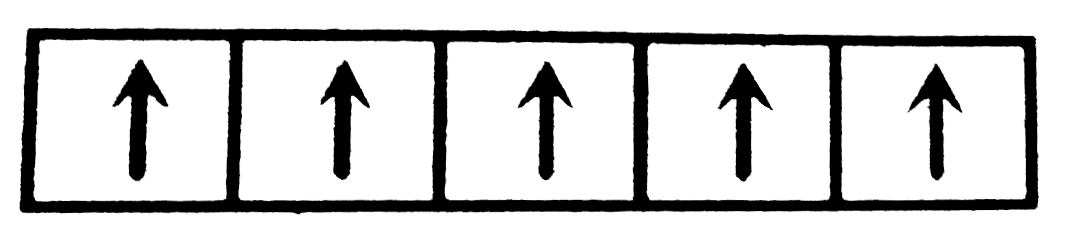
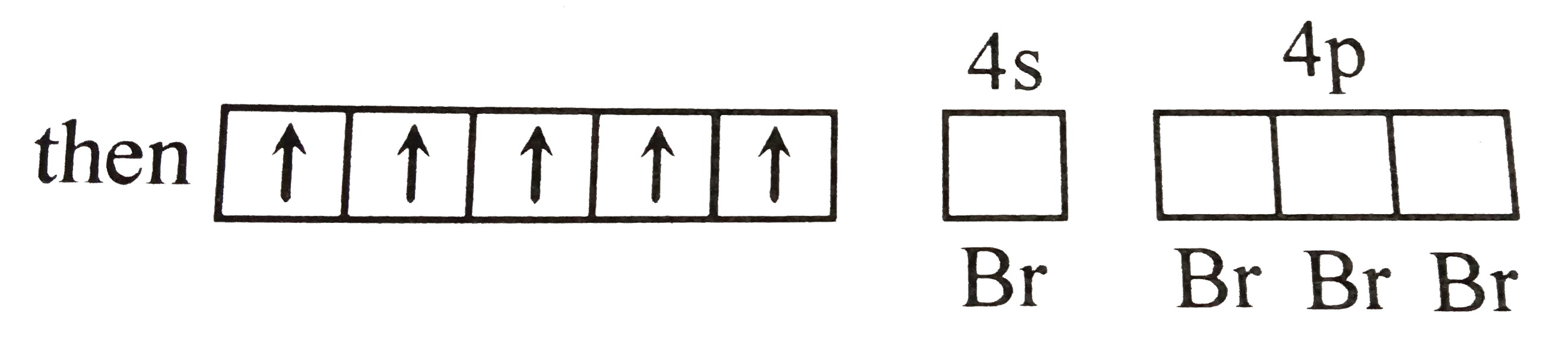
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The geometry and the number of unpaired electron (s) of ["MnBr"(4)]^(2...
Text Solution
|
- Nickel (Z=28) combines with a uninegative monodentate ligand X^(-) to ...
Text Solution
|
- The geometry and the number of unpaired electron (s) of ["MnBr"(4)]^(2...
Text Solution
|
- The number of unpaired electron in respectively are :
Text Solution
|
- Number of unpaired electrons in Mn^(4+) is
Text Solution
|
- [NiCl(4)]^(2-) में अयुग्मित इलेक्ट्रॉनों की संख्या -
Text Solution
|
- Nickel (Z= 28) combines with a uninegative monodentate ligand X^(-) to...
Text Solution
|
- The number of unpaired electrons in [CoCl(4)]^(2-) , [Ni(CO)(4)] and [...
Text Solution
|
- The number of unpaired electrons in [NiCl(4)]^(2-), Ni(CO)(4) and [Cu(...
Text Solution
|