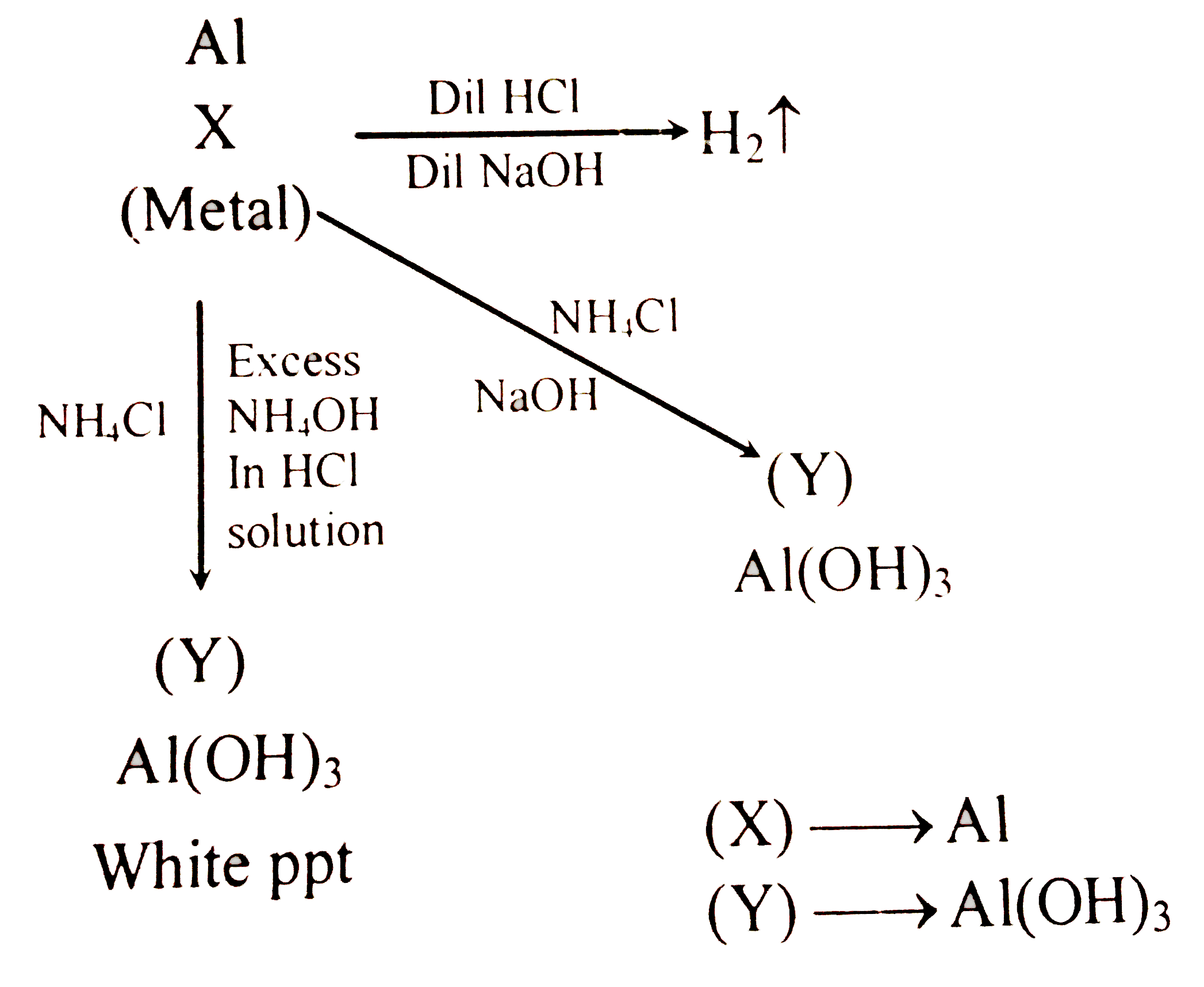A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A metal (X) dissolves both in HCl and dilute NaOH to liberate H(2). Ad...
Text Solution
|
- An inorganic Lewis acid (X) shows the following reactions : (a) It fum...
Text Solution
|
- A solution when diluted with H(2)O And bolled gives a white precipitat...
Text Solution
|
- Why do we add excess of NH(4)Cl and NH(4)OH in the precipitation of gr...
Text Solution
|
- Volume of 2 M HCl required to neutralise the solution containing 1"mol...
Text Solution
|
- A solution when diluted with H(2)O and boiled, it gives a white precip...
Text Solution
|
- NH(4)Cl on heating with NaOH liberates
Text Solution
|
- A metal (X) dissolves both in HCl and dilute NaOH to liberate H(2). Ad...
Text Solution
|
- A white precipitate is obtained when a solution is diluted with H(2)O ...
Text Solution
|