A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-KVPY-PART-1 CHEMISTRY
- The amount (in mol) of bromoform (CHBr(3)) produced when 1.0 mol of ac...
Text Solution
|
- The following compound can readily be prepared by Williamson ethe...
Text Solution
|
- X and Y are
Text Solution
|
- The hyperconjugative stabilities of tert-butyl cation and 2-butene, re...
Text Solution
|
- Benzaldehyde can be converted to benzyl alcohol in concentrated aqueou...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Among the following species, the H-X-H angle (X=B,N or P ) follows the...
Text Solution
|
- The ionic radii of Na^(+),F^(-),O^(2-),N^(3-) follow the order
Text Solution
|
- The oxoacid of phosphorus having the strongest reducing property is
Text Solution
|
- Among C, S and P the element (s) that produces (s) SO(2) on reaction w...
Text Solution
|
- The complex that can exhibit linkage isomerism is
Text Solution
|
- The tendency of X in BX(3)(X=F,Cl,OMe, NMe) to form a pi bond with bor...
Text Solution
|
- Consider the following statement about Langmuir isotherm : (i) The f...
Text Solution
|
- Among the following , the plot that correctly represents the conductom...
Text Solution
|
- The correct representation of wavelength intensity relationship of an ...
Text Solution
|
- The pressure (P)- volume (V)isotherm of a van der Waals gas, at the te...
Text Solution
|
- A buffer solution can be prepared by mixing equal volumes of
Text Solution
|
- The plot of total vapour pressure as a function of mole fraction of th...
Text Solution
|
- On complete hydrogenation, natural rubber produces
Text Solution
|
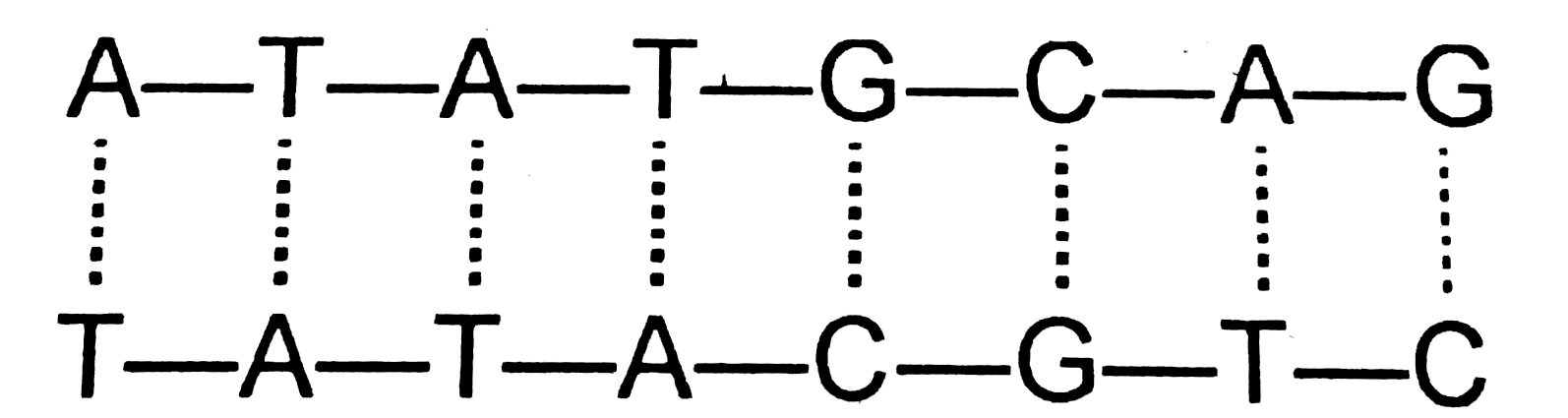
- The average energy of each hydrogen bond in A-T pair is x kcal mol^(-1...
Text Solution
|