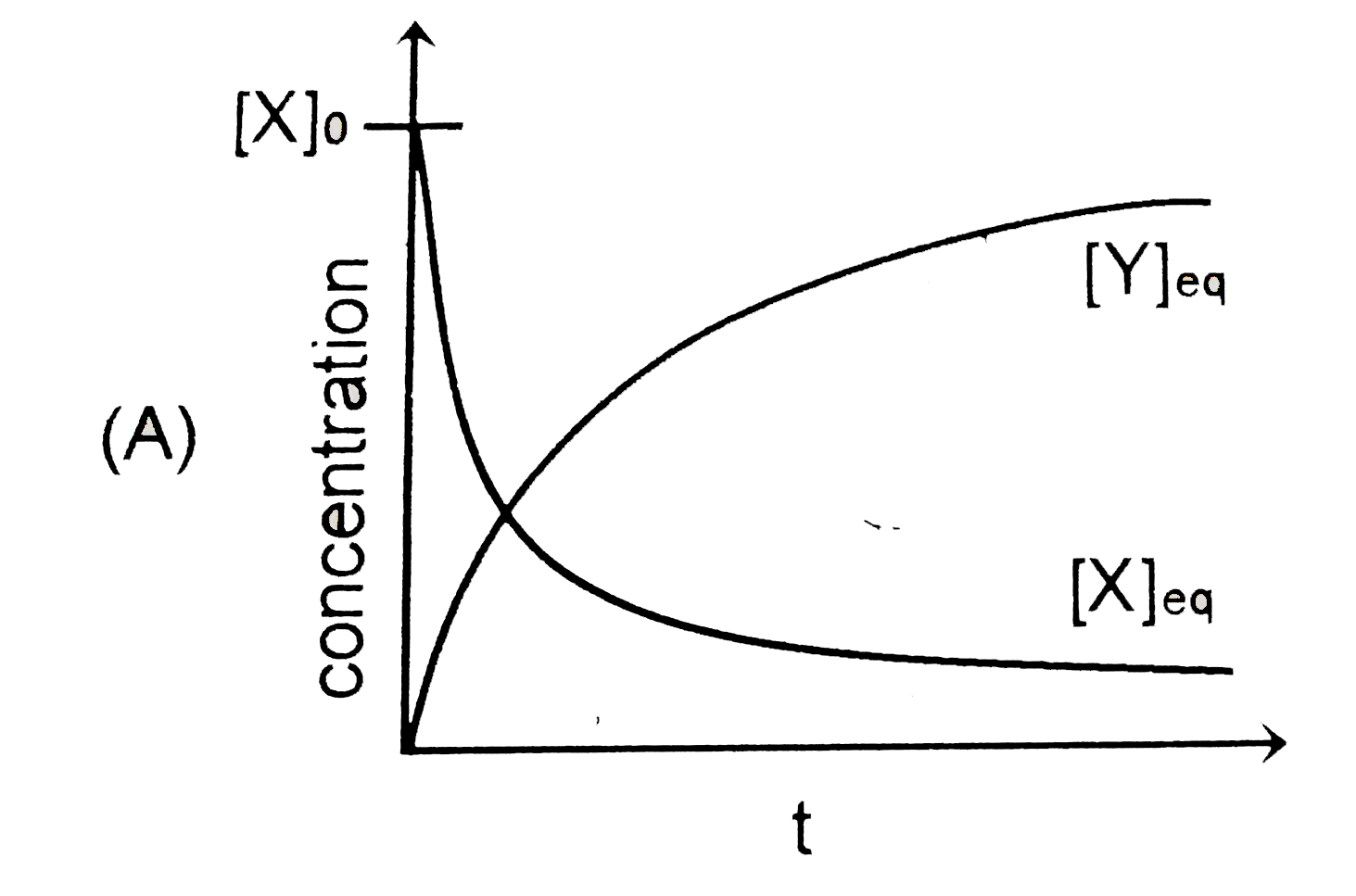
A
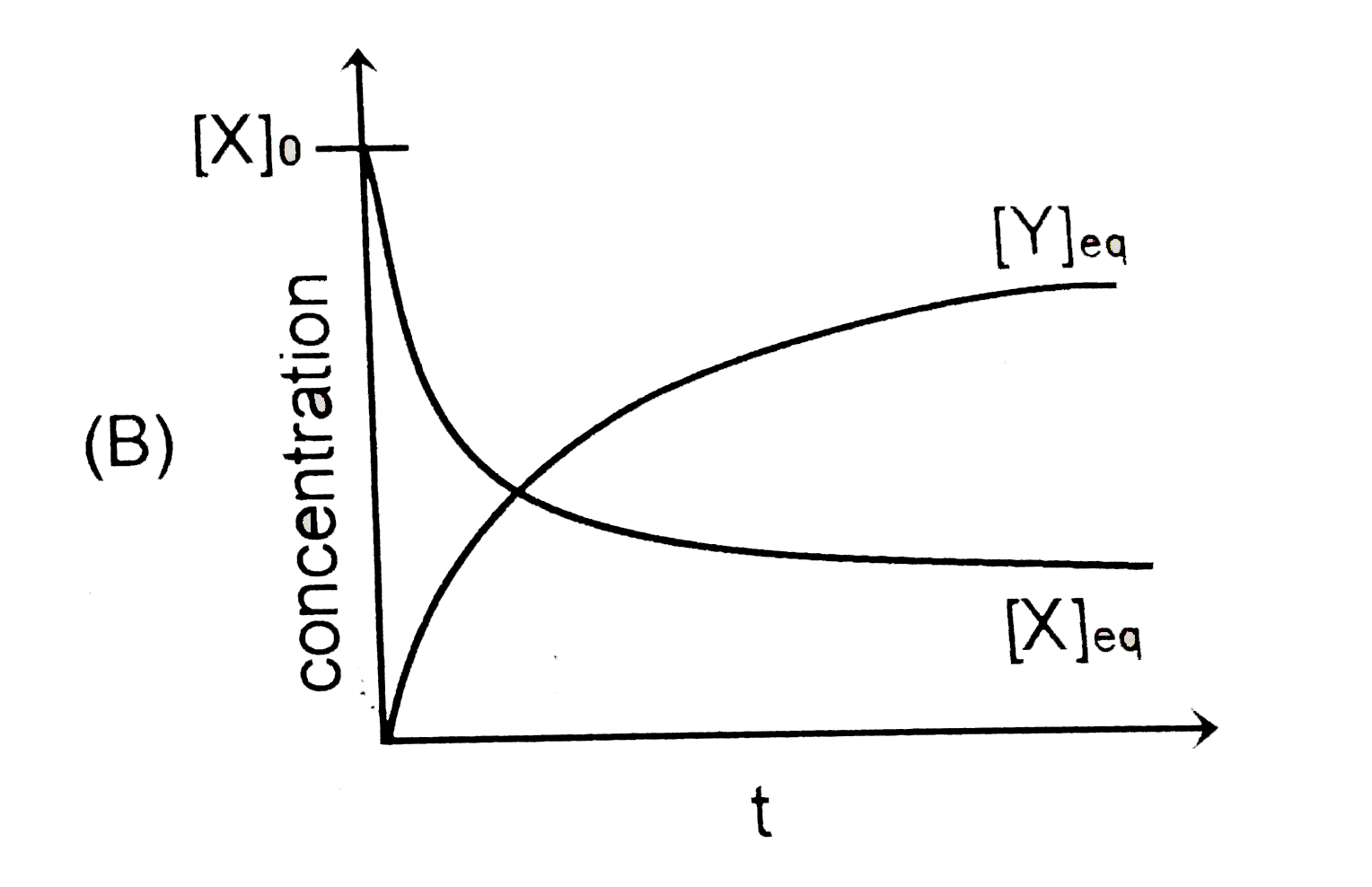
B
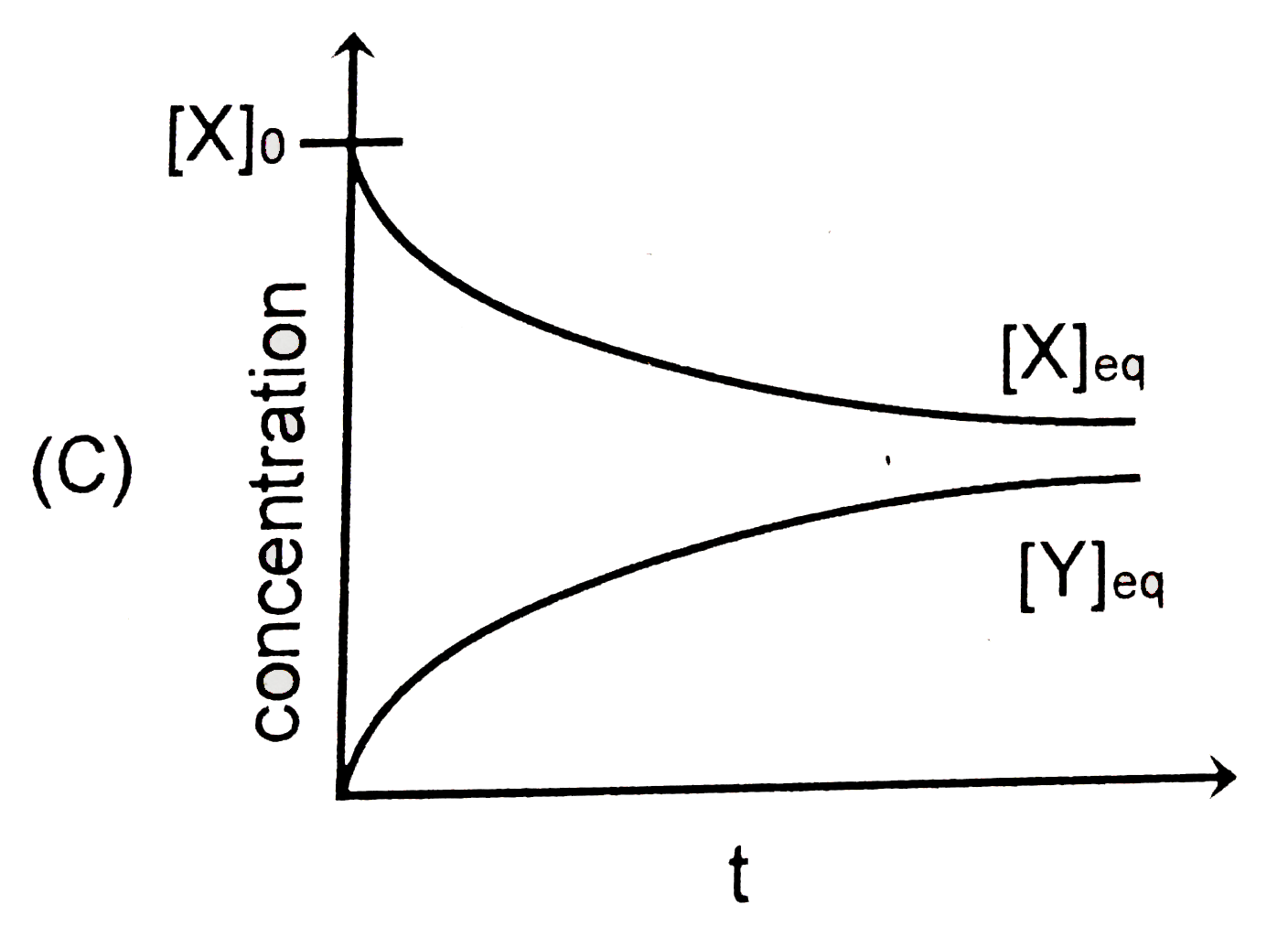
C
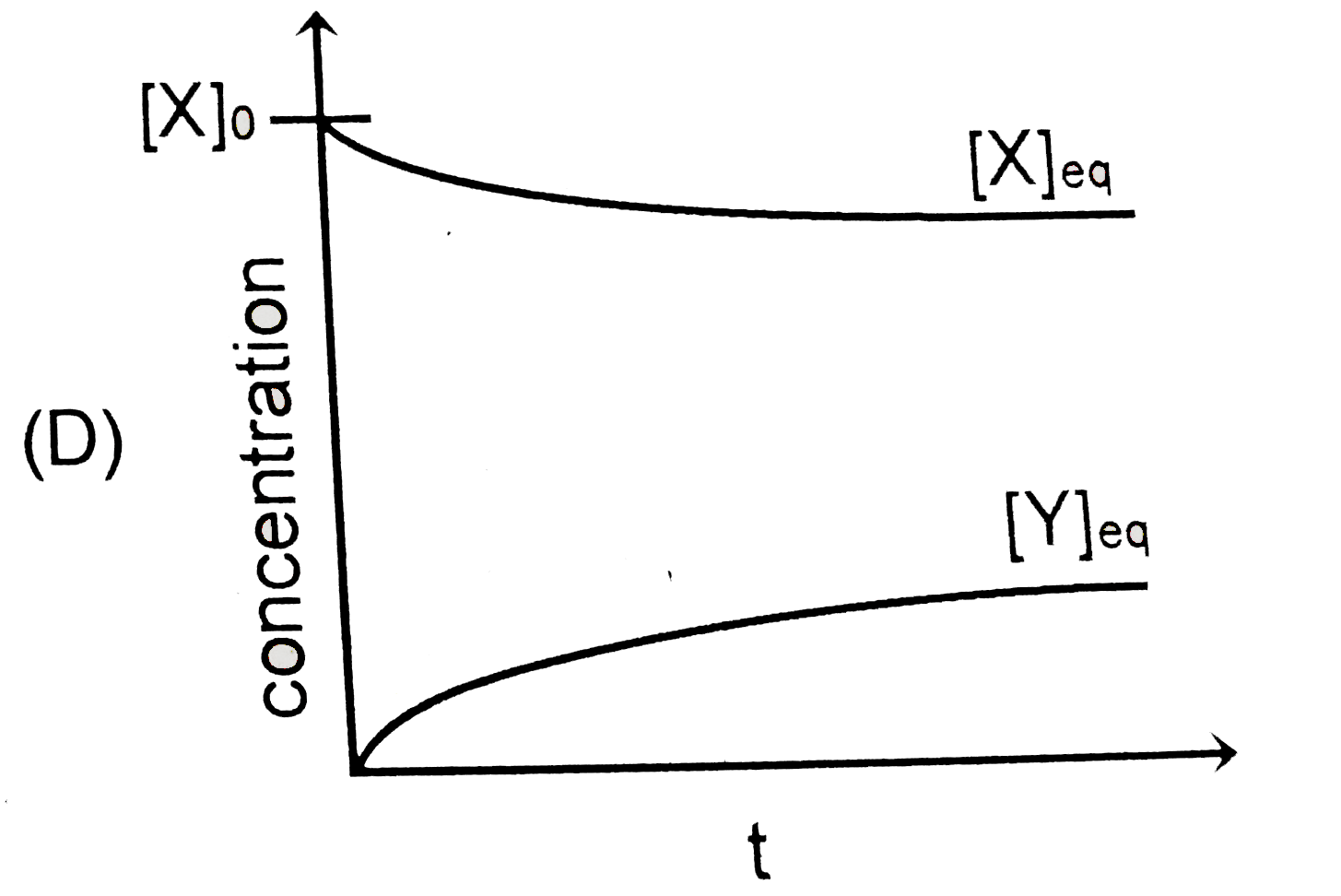
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-KVPY-PART-2 CHEMISTRY
- Upon fully dissolving 2.0 g of a metal in sulfuric acid, 6.8 g of the ...
Text Solution
|
- Upon mixing equal volumes of aqueous solutions of 0.1 M HCl and 0.2 M ...
Text Solution
|
- The products X and Y in the following reaction sequence are-
Text Solution
|
- A pot of the kinetic energy (1//2mv^(2)) of ejected electrons as a fun...
Text Solution
|
- The number of moles of Br(2) produced when two moles of potassium perm...
Text Solution
|
- For the electrochemical cell show below Pt|H(2)(P=1 atm|H^+(aq., x M...
Text Solution
|
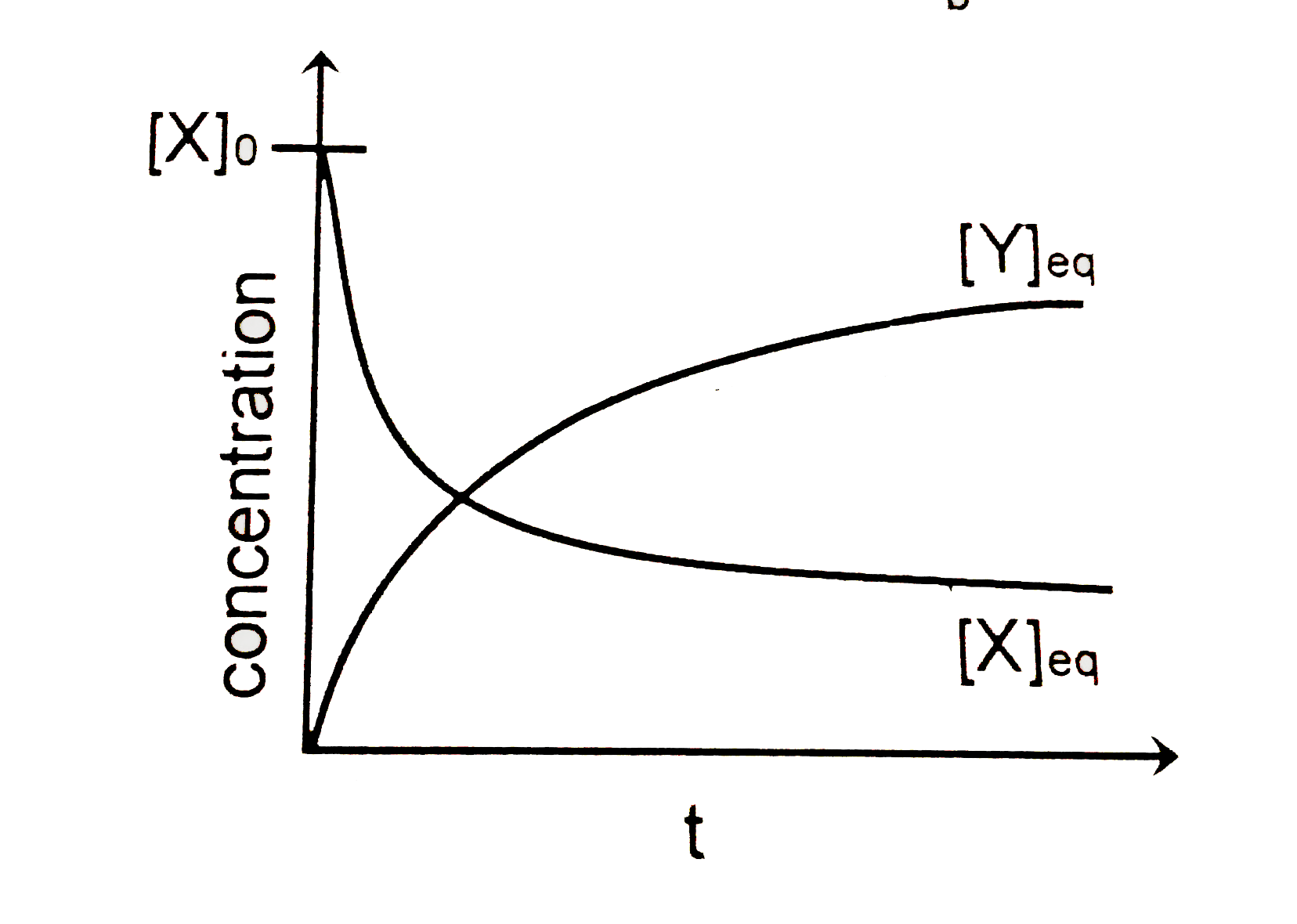
- Consider the following reversible first - order reaction of X at an in...
Text Solution
|
- Nitroglycerine (MW =227.1) denotes according to the following equatio...
Text Solution
|
- The heating of (NH(4))2Cr(2)O(7) produces another chromium compound al...
Text Solution
|
- The complex having the highest spin-only magnetic moment is
Text Solution
|
- Among Ce(4f^(1)5d^(1)6s^(2)), Nd(4f^(4)6s^(2)),Eu(4f^(7)6s^(2))" and "...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Among the following reactions ,a mixture of diastereomers is produced ...
Text Solution
|
- Reaction of phenol with NaOH followed by heating with CO(2) under high...
Text Solution
|
- Tetrapeptide is made of naturally occuring alanine , serine, glycine a...
Text Solution
|