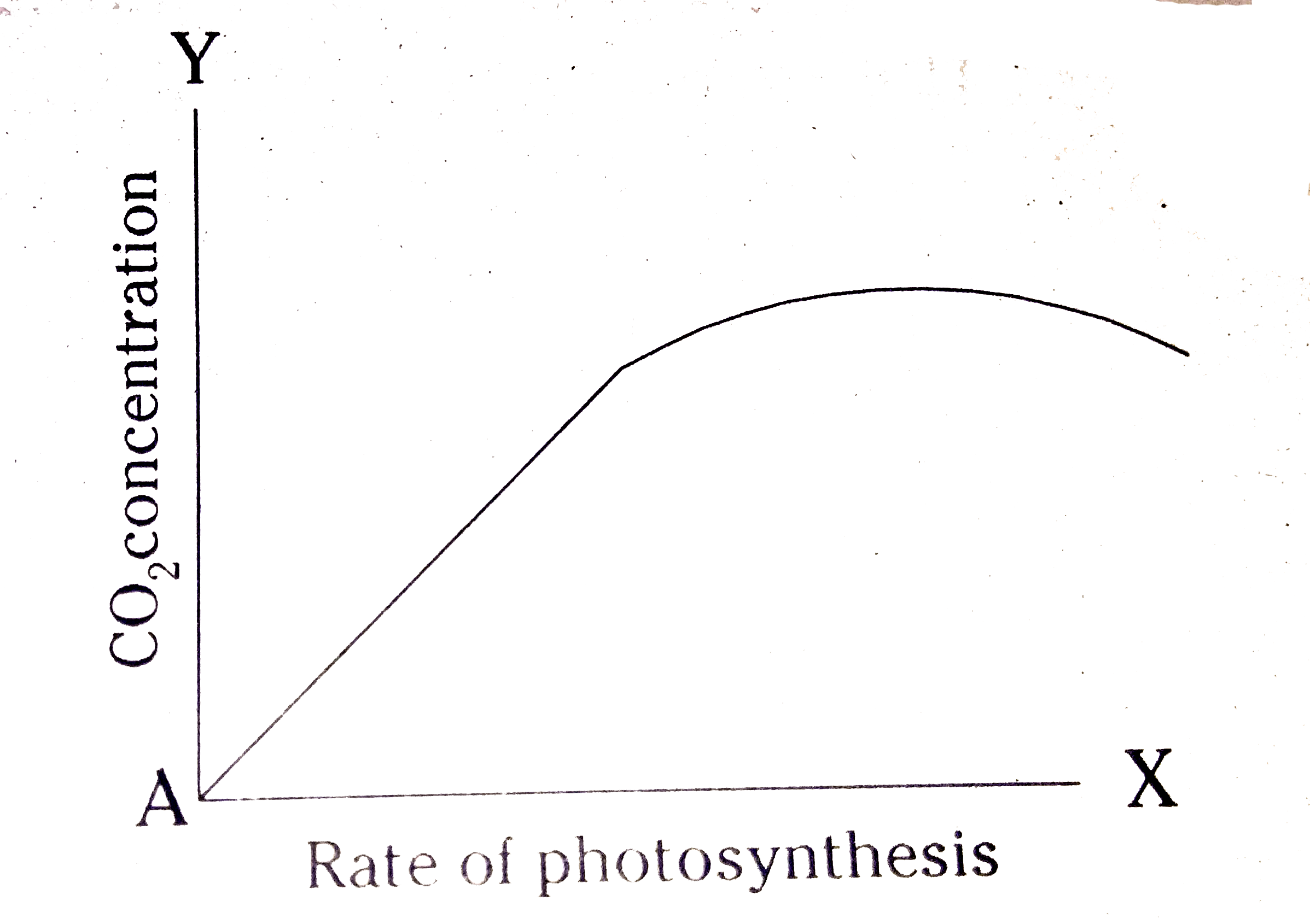Text Solution
Verified by Experts
Topper's Solved these Questions
NUTRITION- FOOD SUPPLYING SYSTEM
VGS PUBLICATION-BRILLIANT|Exercise IMPROVE YOUR LEARNING (EXPERIMENTATION AND FIELD INVESTIGATION)|7 VideosNUTRITION- FOOD SUPPLYING SYSTEM
VGS PUBLICATION-BRILLIANT|Exercise IMPROVE YOUR LEARNING (INFORMATION SKILLS AND PROJECTS)|1 VideosNUTRITION- FOOD SUPPLYING SYSTEM
VGS PUBLICATION-BRILLIANT|Exercise IMPROVE YOUR LEARNING (CONCEPTUAL UNDERSTANDING)|34 VideosNUTRITION
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|446 VideosOBSERVING 4 'R' S
VGS PUBLICATION-BRILLIANT|Exercise CHOOSE THE CORRECT ANSWERS|3 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-NUTRITION- FOOD SUPPLYING SYSTEM-IMPROVE YOUR LEARNING (ASKING QUESTIONS AND MAKING HYPOTHESIS)
- How do non-green plants such as fungi and bacteria obtain their nouris...
Text Solution
|
- If we keep on increasing CO2 concentration in the air, what will be th...
Text Solution
|
- What happens to plant if the rate of respiration becomes more than the...
Text Solution
|
- Why do you think that carbohydrates are not digested in the stomach ?
Text Solution
|