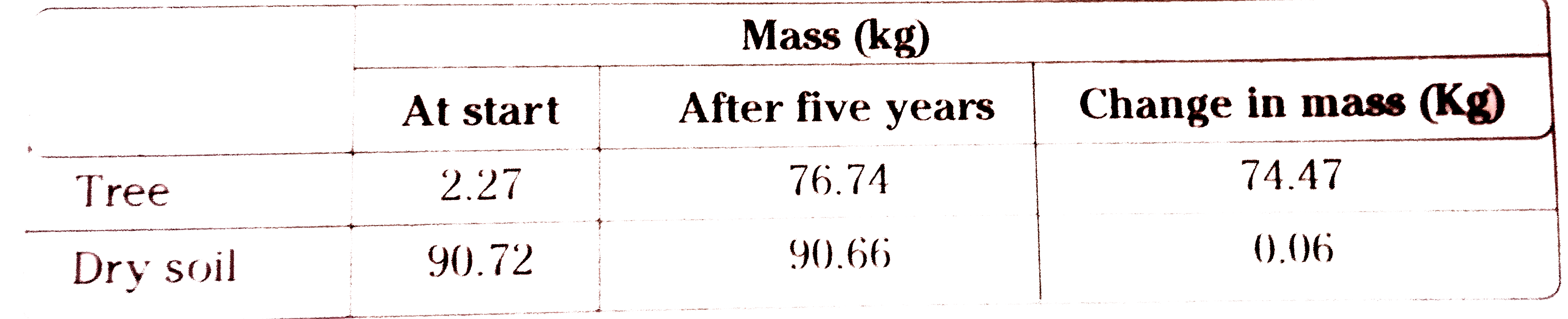Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-NUTRITION- FOOD SUPPLYING SYSTEM-CREATIVE QUESTIONS FOR NEW MODEL PAPER (4 MARK QUESTIONS)
- Differentiate between the nutrition process of plants and animals.
Text Solution
|
- List out the organs/systems involved in the process of digestion and e...
Text Solution
|
- If you have chance to meet any doctor (physician) or nutritionist, wha...
Text Solution
|
- I found that water was essential for the increase of plant mass. Name ...
Text Solution
|
- Write a note on Von Helmont's experiment focusing on how he concluded ...
Text Solution
|
- Roots not only absorb fluid from soil, but returns a portion of their ...
Text Solution
|
- Give an account of water soluble vitamins, their occurrence, deficienc...
Text Solution
|
- Give an account of water soluble vitamins, their occurrence, deficienc...
Text Solution
|
- Observe the information and answer the following questions. What ...
Text Solution
|
- Observe the information and answer the following questions. What ...
Text Solution
|
- Observe the information and answer the following questions. At wh...
Text Solution
|
- Observe the information and answer the following questions. Why c...
Text Solution
|
- Observe the information and answer the following questions. Where...
Text Solution
|
- Write the experimental procedure to prove that oxygen is produced duri...
Text Solution
|
- Draw a neat labelled diagram of T.S. of leaf. What is the function of ...
Text Solution
|
- Deekshith conduced an experiment to find the presence of starch in lea...
Text Solution
|
- Which issues do you take into consideration to tell that plants play a...
Text Solution
|
- We know that by taking different types of food materials we will get v...
Text Solution
|
- Describe what disaster occurs on earth, if photosynthesis life process...
Text Solution
|
- What are vitamins ? Why they are called essential nutrients ? What is ...
Text Solution
|