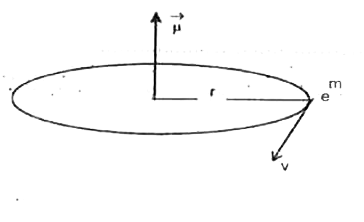
The orbiting electron behaves as a current loop.The equivalent current ,`i=(net charge)/(time of revolution)=(e)/(f)=ef(rot)`.Now, magnetic diople moment `mu=niA`, n=number of the turns of the loop=1,A=area of the loop=`pi r^(2)`,`implies mu =(1)(ef_(rot)) (pi r^(2)) implies mu=pi er^(2) f_(rot)` putting the values of `f_(rot)` and r we obtain,`mu=pie((n^(2)h^(2)epsilon_(0))/(pime^(2)Z))((mZ e^(4)))/(4(epsilon)_(0)^(2)h^(3)n^(3)=(ehn)/(4pim)`