A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
OP TANDON|Exercise Solved Examples|10 VideosATOMIC STRUCTURE
OP TANDON|Exercise Some Solved Examples|12 VideosATOMIC STRUCTURE
OP TANDON|Exercise Integer Answer Type Questions|6 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 VideosCHARACTERISATION OF ORGANIC COMPOUNDS
OP TANDON|Exercise Passage-2|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ATOMIC STRUCTURE -Illustrations of Objective Question
- The number of spectral lines that are possible when electrons in 7th s...
Text Solution
|
- The ratio of radii of first orbits of H, He^(+) and Li^(2+) is:
Text Solution
|
- The energy of second orbit of hydrogen is equal to the energy of ,
Text Solution
|
- What is the energy in eV requried to excite the electron from n=1 to n...
Text Solution
|
- An electron in an atom jumps in such a way that its kinetic energy cha...
Text Solution
|
- If the kinetic energy of an electron is increased 4 times, the wavelen...
Text Solution
|
- The mass of photon having wavelength 1 nm is :
Text Solution
|
- The de Broglie wavelenth of 1 mg grain of sand blown by a 20 m s^-1 wi...
Text Solution
|
- In an atom, an electron is moving with a speed of 600 m//s with an ac...
Text Solution
|
- Velocity of de Broglie wave is given by :
Text Solution
|
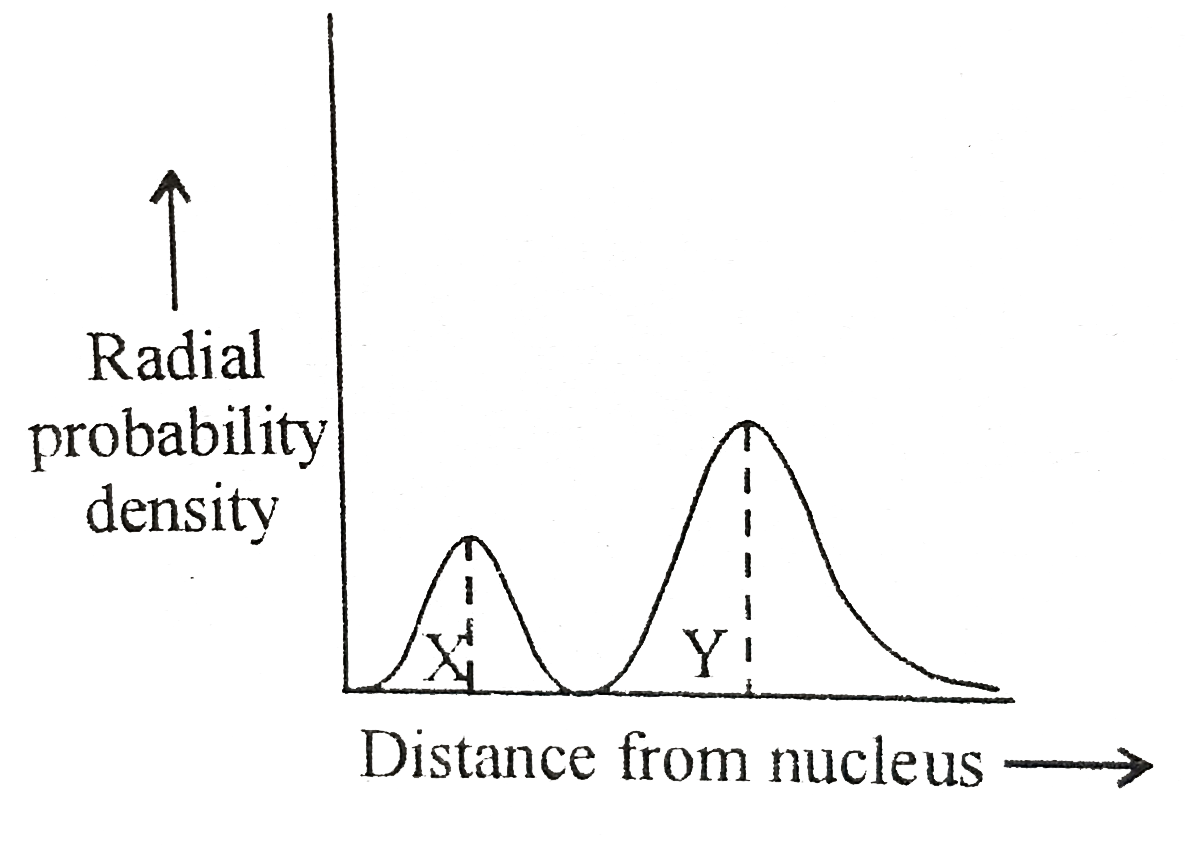
- If the above radial probaility curve indicates '2s' orbital, the dista...
Text Solution
|
- Plots for 2s orbital are X, Y and Z respectively.
Text Solution
|
- The Wave function (Psi) of 2s is given by: Psi(2s)=(1)/(2sqrt2pi)(1/...
Text Solution
|
- The wave function for 1s orbital of hydrogen atom is given by: Psi(1...
Text Solution
|
- The radial wave equation for hydrogen of radial nodes from nucleus are...
Text Solution
|
- The orbital angular momentum of an electron in a d-orbital is:
Text Solution
|
- Which of the following sets of quantum numbers is correct for an elect...
Text Solution
|
- Match the List -I and List-II and select the correct set from the foll...
Text Solution
|
- Which of the following is not possible?
Text Solution
|
- What is the maximum number of electron in an atom that can have the qu...
Text Solution
|