Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
OP TANDON|Exercise Miscellaneous Numerical Examples|12 VideosATOMIC STRUCTURE
OP TANDON|Exercise Practice Problems|88 VideosATOMIC STRUCTURE
OP TANDON|Exercise Solved Examples|10 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 VideosCHARACTERISATION OF ORGANIC COMPOUNDS
OP TANDON|Exercise Passage-2|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ATOMIC STRUCTURE -Some Solved Examples
- The minimum energy requried to overcome the attractive forces between ...
Text Solution
|
- Let a light of wavelength lamda and intensity 'I' strikes a metal surf...
Text Solution
|
- how many orbits, orbitals and electrons are there in an atom having at...
Text Solution
|
- A neutral atom has 2K electrons, 8L electrons and 6M electrons. Predic...
Text Solution
|
- Write down the values of quantum numbers of all the electrons present ...
Text Solution
|
- (a) An electron is in 5f-orbital. What possible values of quantum numb...
Text Solution
|
- Atomic number of sodium is 11. Write down the four quantum numbers of ...
Text Solution
|
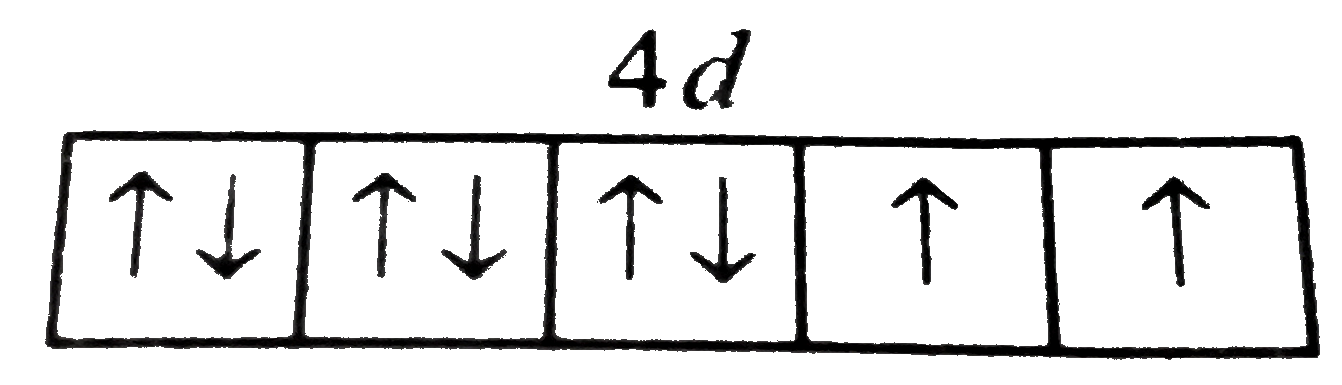
- An element has 8 electrons in 4d-subshell. Show the distribution of 8 ...
Text Solution
|
- How many elements would be in the third period of the periodic table i...
Text Solution
|
- The binding energy of .(2)^(4)He is 28.57 MeV. What shall be the bin...
Text Solution
|
- Calculate the binding energy of the oxygen isotope .(8)^(16)O. The mas...
Text Solution
|
- There are four atoms which have mass numbers 9,10,11 and 12 respective...
Text Solution
|